Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Revista de Cirurgia e Traumatologia Buco-maxilo-facial
versão On-line ISSN 1808-5210
Rev. cir. traumatol. buco-maxilo-fac. vol.11 no.3 Camaragibe Jul./Set. 2011
Immunolocalization of bmp2/4, tgfβ-1 and osteonectin in fibro-osseous lesions of the jaws
Imunolocalização de bmp2/4, tgfß-1 e osteonectina em lesões-fibro-óssea benignas
Ana Paula Veras SobralI; Adriana EtgesII; Fabrício Bitu SousaIII; Ney Soares De AraújoIV; Fábio Daumas NunesIV
I Oral Pathology Department, School of Dentistry, State University of Pernambuco
II Oral Pathology Department, School of Dentistry, Federal University of Pelotas
III Oral Pathology Department, School of Dentistry, Federal University of Ceará
IV Oral Pathology Department, School of Dentistry, University of São Paulo
RESUMO
Objetivo: as lesões fibro-ósseas benignas (LFOB) correspondem a um grupo diverso de patologias caracterizadas pela substituição do tecido ósseo por tecido conjuntivo e matriz extracelular mineralizada. Pouco se conhece a respeito da etiologia desse grupo de lesões. Propomo-nos a analisar por meio da técnica imunohistoquímica a expressão de 3 moléculas (osteonectina, TGFβ-1 e BMP 2/4) envolvidas no metabolismo ósseo. Métodos: Trinta e dois casos diagnosticados como osso normal (ON,8), displasia fibrosa (DF,8), displasia cemento-óssea (DCO,8) e fibroma cemento-ossificante (FCO,8) foram selecionados. Resultados: A osteonectina e a BMP2/4 foram positivas em todos os casos. O TGFβ-1 revelou positividade em 1 caso de DCO e FCO. Conclusão: Os achados imunohistoquímicos sugerem que as LFOB tem processos diferentes de produção de tecido ósseo.
Descritores: Lesões Fibro-ósseas-benignas; Displasia Fibrosa; Displasia Cemento-óssea; Fibroma cementoossificante; BMP2/4, TGFβ-1; Osteonectina; Imunohistoquímica.
ABSTRACT
Background: Benign fibro-osseous lesions (BFOL) comprise a diverse group of pathologies characterized by the replacement of normal bone by fibrous tissue and a mineralized product. Little is known about the biology of this group of lesions. We have analyzed the immunohistochemical expression of three molecules involved in bone metabolism, namely osteonectin, TGF-1, and BMP2/4. Methods: Thirty-two cases diagnosed as normal jaw bone (NJB, 8 cases), fibrous dysplasia (FD, 8 cases), cemento-osseous dysplasia (COD, 8 cases), and cemento-ossifying fibroma (COF, 8 cases) were selected. Results: Osteonectin and BMP2/4 antibodies were positive in all cases. TGF-1 labeling was seen in one case of COD and COF. Conclusion: The immunohistochemistry findings suggest that BFOL have different processes of osseous tissue production.
Keywords: Benign fibro-osseous lesions, Fibrous dysplasia, Cemento-osseous dysplasia, Cementoossifying fibroma, BMP2/4, TGF-1, Osteonectin, Immunohistochemistry.
INTRODUCTION
Benign fibro-osseous lesions (BFOL) comprise a diverse group of pathologies characterized by replacement of normal bone by fibrous tissue and a mineralized product. Despite the advances in our understanding of these conditions, BFOL continue to present problems in classification, diagnosis and management. Since histological findings alone may be similar for lesions with diverse behavioral characteristics and prognosis, in the absence of good clinical and radiological information, the pathologist can only state that a given biopsy is consistent with a BFOL1,2,3,4.
These lesions were classified in three distinct groups; I - fibrous dysplasia, II - reactive lesions arising in the tooth-bearing area, and III - fibro-osseous neoplasms. However, it must be admitted that some cases still defy exact classification1.
Recent advances in biological research have brought the search for markers that can help to classify, characterize or understand the behavior of groups of pathologies. These markers include a variety of proteins usually including growth factors, and hormones among others.
A complex extracellular matrix with collagenous and non-collagenous proteins composes mineralized tissue. Three non-collagenous proteins, osteonectin, transforming growth factor β-1 (TGF-β1) and bone morphogenetic proteins (BMPs), have drawn much attention in the literature lately. Osteonectin has been demonstrated immunocytochemically in active, bone-matrix-producing cells (osteoblasts and young osteocytes)5. Bone morphogenetic proteins (BMPs) form a unique group of proteins within the transforming growth factor beta (TGF-β) superfamily. BMPs were first identified by Urist (1984)6 and there is extensive evidence in support of their role as regulators of bone formation, especially BMP2 and BMP4, which exert potent prodifferentiation effects on early osteoblasts formation. TGF-β1 is a multifunctional regulator of cell growth which will either stimulate or inhibit proliferation of mesenchymal cells depending on the presence of other growth factors7,8,9. TGF-β1 is secreted by osteoblasts and is very abundant in bone matrix10.
Since osteonectin, TGF-β1, and BMP2/4 are produced by osteoblasts we have studied their expression by immunohistochemistry in BFOL in an attempt to have a better comprehension on this group of lesions.
MATERIAL AND METHOD
Thirty two cases diagnosed as normal jaw bone (NJB, 8 cases), fibrous dysplasia (FD, 8 cases), cemento-osseous dysplasia (COD, 8 cases), and cemento-ossifying fibroma (COF, 8 cases) were selected from the archives of the Oral Pathology Department, School of Dentistry, University of São Paulo. Diagnoses were based on a combination of clinical, radiographic and histopathologic information.
All specimens were fixed in 10% neutral formalin, briefly demineralized in 20% formic acid and embedded in paraffin. Three μm sections slides were submitted to immunohistochemistry reactions with anti-osteonectin (Larry W. Fisher, Matrix Biochemistry Unit/NIH, Bethesda, MD, USA, 1:300, 60'), anti-TGF-β1 (Santa Cruz, 1:1000, overnight), and BMP2/4 (Santa Cruz, 1:700, 45') using streptavidin-biotin method. Antigen retrieval methods for each antibody were respectively 0.02% trypsin, 4% pepsin and microwave in EDTA. Human sections of pulp, epithelium, and a stage 20-mouse embryo were used as positive control for osteonectin, TGF-β1 and BMP2/4 antibodies, respectively. As negative control all primary antibodies were substituted with PBS.
RESULTS
Histological analysis has showed that the amount of bone versus cellular fibrous connective tissue stroma varied between groups of lesions. Cemento-osseous dysplasia (COD) and cemento-ossifying fibroma (COF) were more cellular than fibrous dysplasia (FD). However, in individual cases this difference was not consistently noticeable. Most lesions of COD and COF presented woven cementum-like material of cellular and acellular spheres. Bony trabeculae with retiform pattern were not common in COD and COF.
Immunoexpression of normal jaw bone for osteonectin and BMP2/4 were both positive in osteoblasts, osteocytes and in osteoid. However, the TGF-β1 showed negative expression.
FD has shown irregularly shaped trabeculae of immature woven bone in a cellular fibroblastic stroma with few occasional giant cells. The bone trabeculae were delicate and not connected to one another, resembling Chinese script writing (Fig. 1A). FD was osteonectin positive in all osteoid matrix secretory cells, mostly in the round and spindle-shaped ones, and in the osteoid itself. Some polyhedral cells dispersed throughout the collagenous matrix were also positive (Fig. 1B). BMP2/4 was found in most polyhedral and in rare spindle-shaped cells. Cells at the periphery, and inside the osteoid and mineralized structures, were also positive (Fig. 1C). TGF-β1 was negative in all cases.


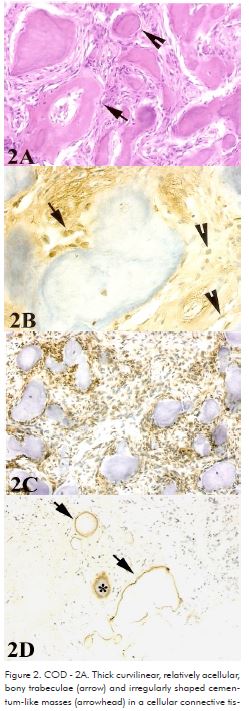
All cases of COD showed thick curvilinear, relatively acellular, bony trabeculae and irregularly shaped cementum-like masses. Thin isolated or retiform trabeculae with prominent osteoblastic rimming were not frequently noted. COD histologic features included a cellular connective tissue stroma, consisting of spindle-shaped fibroblastic cells interspersed by bundles of collagen fibers, numerous small blood vessels, and sporadic hemorrhagic areas (Fig. 2A). Osteonectin labeling was more intense in cells at the trabeculae periphery (Fig. 2B). BMP2/4 was positive in most cells of the lesion, including spindle-shaped and polyhedral cells (Fig. 2C). TGF-β1 antibody has labeled cells in the center and periphery of mineralized material in just one case (Fig. 2D).
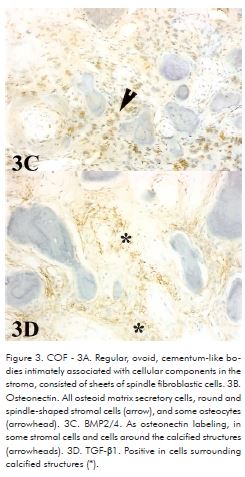
COF cases showed regular, ovoid, cementum-like bodies intimately associated with cellular components in the stroma. The connective tissue consisted of sheets of spindle fibroblastic cells with occasional areas of storiform pattern (Fig. 3A). COF were positive to osteonectin in all osteoid matrix secretory cells, round and spindle-shaped stromal cells, including matrix itself, and some osteocytes (Fig. 3B). BMP2/4 labeling was found in some cells and cells around the calcified structures (Fig. 3C). TGF-β1 was positive in cells surrounding calcified structures in only one case (Fig. 3D).

DISCUSSION
Bone marrow stromal cells form an important source of pluripotential progenitors that are capable of differentiating into osteoblasts under the appropriate conditions. Osteoprogenitor cells have no morphological characteristics to distinguish them from other mesenchymal cells. There is still a need for clarification of many aspects of this perplexing group of lesions11. That is one of the reasons why antigenic markers for the differentiation stages of these cells, will probably help in distinguishing between groups of mineralization producing lesions. Some recent progress has been made in identifying early markers for the osteoblast lineage as definition of the osteoprogenitor phenotype12. Osteonectin, BMPs and the growth factor TGF-β are well known participants in the osseous tissue development, however there are no reports of their participation in BFOL.
Our result showed that osteonectin, an osteoinductor protein, is expressed in all studied lesions with differences between the groups. Jundt et al. (1989)13 found osteonectin in active osteoblasts and osteoprogenitor cells as well as in young osteocytes, while aged, quiescent osteocytes did not contain the protein. We encountered a positive staining to osteonectin in polyhedral, osteoblast-like cells, spindle-shaped and round cells, adjacent to newly formed woven bone, and cells located within the trabeculae of bone. The former cells may be considered quiescent osteocytes, and, if so, these different results may be because a distinct antibody was used. We also saw antibody positivity in the osteoid matrix of FD (data not shown). Schulz et al. (1988)5 states that osteonectin is an important and helpful tool for establishing the definite diagnosis of osteoprogenitor cell tumours. However, Bosse et al. (1990)14 have shown osteonectin expression in mesenchymal cells tumours with no bone production, suggesting that this protein is not specific enough to serve as a differential marker against other bone mesenchymal tumours. Our findings suggested that osteonectin may be linked to cells presenting an osseous matrix secretory potential. As the Sakamoto et al. (1999)15, we observed no osteonectin expression in bone matrix, confirming the presence of mature bone in FD. In the COF cases a positive expression of osteonectin was observed in most spindle-shaped cells of the stroma. This fact may be related to the high potential to form mineralized tissues seen in these lesions.
In FD, BMP was positive in polyhedral and rare spindle-shaped cells, including those at the periphery of osteoid and mineralized structures. All FD cases were negative to TGF-β1. Yan and Lianjia (1990)16 found in FD of bone, that BMP was abundant in the fibrocellular tissue with osteogenic activity. In contrast, fibrous tissue of COF showed a weak positive staining, and only the osteoblasts rimming the bone were positive. Because of this staining pattern the authors considered that it was possible to differentiate ossifying fibroma from fibrous dysplasia of bone by immunohistochemistry. Our findings are different from these authors in the sense that BMP, as the two other markers, does not differentiate the lesions studied, especially when these lesions form osseous trabeculae. However, it is reasonable to consider that BMP should participate in the induction of newly formed mineralized structures of FD, COD and COF, since it has been shown to induce heterotopic bone formation in various animal species and to produce growth stimulation effect on bone17.
Since TGF-β1 was positive in only one case of COD and COF, determining the expression pattern of this antibody was not possible. TGF-β1 promotes formation of new woven bone and stimulates fracture repair following injection into the periosteum18. Expression of TGF-β isoforms in osteosarcomas has shown that especially TGF-β3 may play a role in osteosarcoma progression19. TGF-β2 is most highly associated with bone and has a higher specific activity for bone induction20. Increased TGF-β1 synthesis provides a significant advantage for tumor formation4. Previous studies have shown that TGF-β2 and TGF-β3 generally were expressed in transitional chondrocytes, whereas TGF-β1 was expressed more highly in areas of osteogenesis3. The findings described in the literature suggest that the TGF-β1 isoform expression is more related to tumor progression of malignant cells. These findings possibly justify the absence of labeling in our cases. It is interesting to note that although TGF-β has been shown to stimulate extracellular matrix production, it inhibits matrix mineralization for reasons that currently are unclear21.
Differentiation of COF, COD and FD is important because of differences in treatment and prognosis. Immunohistochemistry together with cellular morphology provided no recognizable pattern allowing to discriminate the lesions studied, however it suggests that they have different processes of osseous tissue production. There is still a need for clarification of many aspects of this perplexing group of lesion. The present findings pave the way for additional approaches to determine the mechanisms of mineralized tissue formation in this group of lesions.
REFERENCES
1. WALDRON, C.A Fibrous-osseous lesions of the jaws. J Oral Maxillofac Surg 1993:51: 828-835. [ Links ]
2. SUN, L.; WEATHERS, D.R.; WALDRON, C.A Distinguishing of focal cemento-osseous dysplasias and cemento-ossifying fibromas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997:84:301-9/540-9.
3. NEVILLE,B.W.; DAMM, D.D.; ALLEN, C.M.; BOUQUOT, J.E. Oral Pathology and Maxillofacial. Philadelphia: W.B. Saunders, 2009: 817.
4. SLOOTWEG, P.J. Maxillofacial fibro-osseous lesions: classification and different diagnosis. Semin Diagn Pathol 1996:13(2):104-12.
5. SCHULZ, A; JUNDT,G.; BERGHÄUSER, K.H; GEHRON-ROEY, P.; TERMINE, J.D. Immunohistochemical study of osteonectin in various types of osteosarcoma. American Journal of Pathology1988:132(2): 233-238.
6. URIST, M.R.;HUO, Y.K.; BROWNELL, A.G.; et al. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatographic. Proc Natl Acad Sci USA 1984:81:371-371.
7. FLANDERS, K.C.; ROBERTS, A B.; FLEURDELYS, B.E.; SPORN, M.B. Antibodies to peptide determinants in transforming growth factor β and their applications. Biochemistry 1988:27:739-746.
8. MOES, H.L.; YANG, E.Y.; PIETENPOL, J.A. TGF-β stimulation and inhibition of cell proliferation: new mechanism insights. Cell 1990:63:245-247.
9. MASSAGUÉ, J. TGF-β signaling: Receptors, transducers, and mad proteins. Cell 1996:85:947-950.
10. NODA, M.; RODAN, G.A. Type β transforming growth factor regulates expression of genes encoding bone matrix proteins. Connective Tissue Research 1989:21:71-75.
11. BRANNON, R.B.; FOWLER, C.B. Benign fibro-osseous lesions: A review of current concepts. Adv Anat Pathol 2001: 8(3):126-143.
12. FRANCESCHI, R.T. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med 1999:10(1):40-57.
13. JUNDT, G.; SCHULZ, A; BERGHÄUSER, K.H., L.W. FISHER, GEHRON-ROBEY, P.; TERMINE, J.D. Immunocytochemical identification of osteogenic bone tumors by osteonectin antibodies. Virchows Archiv A Pathol Anat 1989:414:345-353.
14. BOSSE, A; VOLLMER, E.; BÖCKER, W.; ROESSNER, A The impact of osteonectin for differential diagnosis of bone tumors: an immunohistochemical approach. Path Res Pract 1990:186:651-657.
15. SAKAMOTO, A.; ODA, Y.; IWAMOTO, Y.; TSUNEYPSHI, M. A comparative study of fibrous dysplasia and osteofibrous dysplasia with regard to expression of c-fos and c-jun products and bone matrix proteins: a clinicopathologic review and immunohistochemical study of c-fos, c-jun, type I collagen, osteonectin, osteopontin, and osteocalcin. Hum Pathol 1999:30(12):1418-26.
16. YAN, J.; LIANJIA,Y. The relationship between bone morphogenetic protein and neoplastic bone diseases. Clinical Orthopaedics and Related Research 1990:259:233-238.
17. LEE, M.B. Bone morphogenetic proteins: background and implications for oral reconstruction. J Clin Periodontol 1997:24:355-365.
18. CHEIFETZ, S.; LI, I.W.S.; McCULLOCH, C.A.G.; SAMPATH, K.; SODEK, J. Influence of osteogenic protein-1 (OP-1; BMP-7) and transforming growth factor- β1 on bone formation In Vitro. Connective Tissue Research 1996:35:1-4/:71:125-132.
19. KLOEN, P.; GEBHARDT, M.C.; PEREZ-ATAYDE, A.; ROSENBERG, A. E.; SPRINGFIELD, D. S.; GOLD, L.I.; MANKIN, H.J. Expression of transforming growth factor-beta (TGF-beta) isoforms in osteosarcomas, TGF-beta 3 is related to disease progression. Cancer 1997:80 (12):2230-2239.
20. CENTRELLA, M.; HOROWITZ M.C., WOZNEY, J.M.; McCARTHY, T.L. Transforming growth factor-beta gene family members and bone. Endocr Rev 1994:15:27-39.
21. CHANG, H.; GILLETT, N.; FIGARI I.; LOPEZ, A.R.; PALLADINO, M.A. DERYNCK, R. Increased transforming growth factor beta expression inhibits cell proliferations in vitro, yet increases tumorigenicity and tumor growth of Meth A sarcoma cells. Cancer Res 1993:53:4391-8.
Acknowledgements
We thank Professor Drª Suzana Cantanhede Orsini Machado de Sousa, Department of Oral Pathology, School of Dentistry, University of São Paulo, for their comments and criticisms. To Edna Toddai and Elisa dos Santos for the excellent technical assistance in the slides confection. Supported by FAPESP grant 99/05383-6 and CNPq grant 46111/00-0.













