Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Revista Odonto Ciência (Online)
versão On-line ISSN 1980-6523
Rev. odonto ciênc. (Online) vol.25 no.4 Porto Alegre Out./Dez. 2010
ORIGINAL ARTICLE
Temperature variation in pulp chamber during dental bleaching in presence or absence of light activation
Variação da temperatura na câmara pulpar durante o clareamento dental na presença ou ausência de fotoativação
Fernanda Brandão Mollica; Daniel Maranha da Rocha; Alessandro Caldas Travassos; Marcia Carneiro Valera; Maria Amélia Maximo de Araujo
Department of Restorative Dentistry, Sao Jose dos Campos Faculty of Dentistry, State Paulista University, Sao Jose dos Campos, SP, Brazil
ABSTRACT
PURPOSE: In addition to the chemical damage due to bleaching gels penetration into the pulp during pulp vitality dental bleaching, another possible aggressive factor could be the heat generated by the exothermal oxidation reaction of the bleaching gel, which may also be aggravated by the use of light activation. This study assessed the temperature variation in the pulp chamber in human teeth, using three different bleaching gels with or without LED light activation.
METHODS: Thirty human pre-molars were cut longitudinally to obtain buccal and lingual halves. The 60 specimens were divided into 3 groups, and the bleaching gel used varied as follows: 35% hydrogen peroxide (WHP); 37% carbamide peroxide (W) and 38% hydrogen peroxide (OX). Half of the specimens were submitted to bleaching with light activation and, the other half, without light activation. The light source used was the light emitting diode appliance (LED, 3-Light, Clean Line), and the intrapulpal temperatures were measured by using a digital thermometer. Data were analyzed by ANOVA and Tukey's tests (alpha=5%).
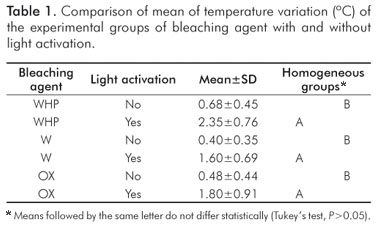
RESULTS: The intrapulpal temperatures (inºC) were as follows: without light activation (WHP= 0.68b; W= 0.40b; OX= 0.48b); with light activation (WHP= 2.35a; W= 1.60a; OX= 1.80a ).
CONCLUSION: LED light activation of bleaching gels increased the temperature in the pulp chamber, but did not achieve the critical temperature value of 5.5ºC.
Key words: Bleaching; activation; temperature; light; peroxide
RESUMO
OBJETIVO: Além da agressão química devido à penetração dos géis clareadores na polpa durante o clareamento de dentes com vitalidade pulpar, outro possível fator agressor pode ser o calor gerado pela reação de oxidação exotérmica do gel clareador, que pode também ser agravada pela fotoativação. Este estudo avaliou a variação da temperatura na câmara pulpar de dentes humanos, utilizando-se três diferentes géis clareadores, com ou sem fotoativação.
METODOLOGIA: Trinta pré-molares humanos foram cortados longitudinalmente para obtenção de duas metades: vestibular e lingual. Os 60 espécimes foram divididos em 3 grupos e os agentes clareadores utilizados variaram como segue: peróxido de hidrogênio 35% (WHP), peróxido de carbamida 37% (W) e peróxido de hidrogênio 38% (OX). Metade dos espécimes foi submetida ao clareamento com fotoativação e, a outra metade, sem fotoativação. A fonte de luz utilizada foi o aparelho à base de diodo emissor de luz (LED, 3-Light, Clean Line) e as temperaturas foram medidas por um termômetro digital. Os dados foram analisados por análise de variância e teste de Tukey (alfa=5%).
RESULTADOS: Os resultados de temperatura foram: sem fotoativação (WHP= 0.68b; W= 0.40b; OX= 0.48b); com fotoativação (WHP= 2.35a; W= 1.60a; OX= 1.80a).
CONCLUSÃO: A fotoativação dos géis clareadores com LED contribuiu para um maior aumento de temperatura na câmara pulpar, mas não se atingiu a temperatura crítica de 5,5ºC.
Palavras-chave: Clareamento; ativação; temperatura; luz; peróxido
Introduction
Dental bleaching has become increasingly outstanding among the esthetic procedures routinely performed by dentists. It is very important to point out that a smile with a healthy appearance significantly increases the patient's self-esteem and confidence and projects an image of health. In addition, studies have proved that the satisfaction with smile is very important in personal and professional's life (1).
The most frequently used substances for bleaching vital teeth are 10% to 37% carbamide peroxide and 1.5% to 38% hydrogen peroxide, with the most concentrated products reserved for professional use in the dental office (2). The great advantage of in-office whitening is the time economy because this high concentration bleaching gel acts faster being able to alter the shade from 8 to12 tones (3). The use of light to enhance the effects of the bleaching gel is recent, and a study have pointed out that this procedure can accelerate dental bleaching process (4), but more recent publications indicate that the benefit of the additional use of light is limited (5).
The light activation units most used at present are the quartz-halogen-tungsten (QHT), argon lasers, plasma arc and light emitting diodes (LEDs), which have the advantage of promoting photo-chemical reactions and later selective and controlled heating of only the gel, instead of the entire dental structure (5). Michida et al. (6) found that light activation with LED promoted less temperature variation in the pulp chamber when compared to halogen light and Nd:YAG laser.
A frequent concern as regards the use of peroxides at high concentrations is their penetrability into dental tissues. Benetti et al. (7) demonstrated that significant amounts of hydrogen peroxide were found in the pulp chamber in teeth bleached with carbamide peroxide at high concentrations. Hanks et al. (8) affirmed that hydrogen peroxide diffusion is higher as the bleaching gel concentration and the time it remains in contact with the tooth increases. According to Leonard et al. (9), clinically hypersensitivity is a result of reversible reactions and it can be observed in both in in-office and home bleaching.
In addition to the chemical action due to bleaching gels penetration into the pulp chamber during dental bleaching with pulp vitality, another possible aggressive factor could be the heat generated by the exothermal oxidation reaction of the bleaching gel (7), which may also be aggravated by the use of light activation. According to Brännström (10,11) the heat causes the liquid in the dentinal tubules to expand, and can lead to vascular injury and tissue necrosis.
In view of this, the aim of this study was to make an in vitro assessment of the temperature variation in the pulp chamber in human teeth, using three different bleaching gels with or without LED light activation.
Methods
Thirty healthy human premolars, extracted due to periodontal problems, were cleaned with periodontal curettes to remove the periodontal ligament remains. The selected teeth were stored in distilled water at 4ºC, exchanged periodically until the time of use, not exceeding a period of 6 months (ISO 11405). Next, the root apexes were included in chemically activated acrylic resin (Jet, Clássico, Sao Paulo, Sao Paulo, Brazil) with the use of a silicone mold (Silibor, Clássico, Sao Paulo, Sao Paulo, Brazil) to enable the teeth to be cut longitudinally, in the mesio-distal direction, just in the middle of the occlusal surface, using a cutting machine under refrigeration (Labcut 1010 Extec, Sao Paulo, Sao Paulo, Brazil), producing a buccal and a lingual segments to facilitate the access to the pulp chamber.
The dental thickness of the buccal and lingual faces was standardized by using a thickness meter, at 2 mm for each segment by wearing down the lateral, buccal or lingual wall of the pulp chamber with a high speed spherical carbide burr (JET, Beavers Dental, Morrisburg, ON, Canada). Each root portion was embedded in acrylic resin.
The 60 specimens obtained were randomly divided into 3 groups (n=20), according to the bleaching gel used: Group WHP: 35% hydrogen peroxide (Whiteness HP 35%, FGM, Joinville, SC, Brazil), Group W: 37% carbamide peroxide (Whiteness Super 37%, FGM, Joinville, SC, Brazil) and Group OX: 38% hydrogen peroxide (Opalescence Xtra Boost, Ultradent, South Jordan, Utah, USA). The bleaching gel was applied over the entire enamel area, according to the manufacturer's instructions.
In each of the groups, half of the specimens were submitted to bleaching with light activation and the other half, without light activation. The light source LED 3-Light (500 mW/cm2, Clean Line, Taubate, SP, Brazil) was applied for 30 s, keeping a distance of 5 mm to the gel surface. Whitening procedures were performed by the same operator.
To measure the temperature variation during the whitening procedure, a digital thermometer MT 507 (Minipa, Sao Paulo, Sao Paulo, Brazil) was used. Room temperature was standardized by an air conditioner unit set at 23ºC±1ºC. Before the experiment began, the specimens were placed in a container with water at room temperature (23ºC±1ºC) so that they would not dry out, what could influence on temperature variation. Thus, the specimens were removed from the container one by one before the whitening procedure began, and lightly dried with an air jet. Next, the pulp chamber was filled with a thermal paste (Implastec, Votorantim Indústria Brasileira, Sao Paulo, SP, Brazil) to improve thermal conductance, and the thermocouple tip was positioned in the pulp chamber as closely as possible to the buccal surface. The thermocouple wire was fixed to the tooth with wax.
The temperature inside the pulp chamber was measured before the bleaching gel application (T0); during light activation of the bleaching gel (T1) and during the color change of the bleaching gel until achieving a clear color (T2). So, it would be possible to verify any increase in temperature even when the light source was not being used anymore, but when there were still happening chemical reactions. The specimens which were not activated followed only the steps T0 and T2.
The temperature variation was calculated by the following equation: maximum temperature value during T1 or T2–T0. Data followed a normal distribution and were analyzed by using a two-way ANOVA and Tukey's test for comparison of means between groups (Mini-Tab 14.12, 2004). A level of significance of 0.05 was set for all tests.
Results
There was a statistically significant difference between acti- vation and no activation groups (P<0.001). LED light acti- vation of bleaching gels increased the temperature (Table 1).
Discussion
This study showed that LED light activation of bleaching gels contributed to greater variation of temperature increase in the pulp chamber, but did not achieve the critical value of 5.5ºC. It must be pointed out that in vitro study models, such as the used in this study, are not capable of reproducing the pulp blood flow, which is capable of dissipating the heat applied (12). So, it should be considered that, in vivo, the temperature rise presents lower values than those found in this study (3). The temperature values measured in this study cannot be directly applied for temperature variations in vivo.
In the present study, the premolar surface thickness up to the pulp chamber was standardized at 2 mm. According to Wetter et al. (4), the size of teeth interferes in temperature increase, since in their study the temperature was lower in the molars than in the incisors and pre molars. Furthermore, Sulieman et al. (3) found out that the smaller teeth, such as the lateral incisors, presented a higher temperature increase when compared with the canines, possibly due to the difference in dentinal thickness between these teeth.
In-office bleaching products are based on hydrogen peroxide applied as a paste or gel on the tooth surface (13). In the bleaching process with hydrogen peroxide, the bleaching gel may or may not be indicated in association with a light and/or heat source. The purpose of the energy supplied is to accelerate hydrogen peroxide degradation and prove an enhanced effect in a shorter clinical time (14).
Although efficient, there have been reports of post-operative sensitivity by patients, as regards whitening procedures, mainly those performed in-office (15). Bowles and Thompson (16) showed that pulp enzymes were inhibited by hydrogen peroxide, especially when the more concentrated agents were used. Tjan and Dunn (13) and Greenwall (17) related that sensitivity could be related to pulp inflamma- tion induced by heat, which could cause irreversible pulp damage if a temperature increase higher than 5.5ºC is generated (18).
In the present study, the condition with activation always presented higher temperature variations for all the bleaching gels used. Also, when the type of bleaching gel was assessed, the mean values with activation differed statistically from the condition without activation. These results were expected as when light is projected onto a bleaching gel, a small fraction is absorbed and its energy is converted into heat. So, the use of light can have a thermal catalytic and a photolytic effect. The first one happens as the release of hydroxyl-radicals from peroxide is accelerated by temperature rise, and the last one is explained through direct excitation of peroxide by light (5).
Although the potential increase in efficacy of bleaching gels by light activation is still not well documented, neither the application of light nor the application of heat seem to increase the decomposition rate of peroxide (19). The use of halogen, LED and plasma arc light were equally efficient to promote hydrogen peroxide decomposition when compared with groups without light activation (20-22). The best results were associated with a longer contact time of the bleaching gel with the tooth, and mainly with a greater number of times that the gel was changed (20). Moreover, some side effects of light activation, such as the temperature increase in the pulp and the increased penetration of peroxide into the pulp chamber should be emphasized (5).
In the present study, the use of light activation significantly increases temperature in the pulp chamber, but no group achieved the critical temperature of 5.5ºC. These findings are in agreement with those by Zanin et al. (23), who used LEDs for activating the bleaching gel with temperature rise of only 2ºC. Although the present study did not show critical temperature variations, it is important to be aware of the possible variations that may occur in the dental pulp. Even small temperature rises can cause an inflammatory process in the pulp tissue, which may be reversible or irreversible depending on the temperature reached and on the duration that the tooth was submitted to heating (17). Application of 35% hydrogen peroxide with heat showed pulp alterations, including obliteration of odontoblasts, hemorrhage, inflammation and internal dentin resorption (24). Clinical data revealed sporadic and reversible reactions both with in-office and home techniques (9).
Although there are defense mechanisms against the irritation of bleaching gels, Leonard et al. (22) emphasized the need for care in the use of bleaching gels. It should be pointed out that even with a temperature rise lower than 5.5 ºC, there is chemical aggression that could contribute to pulp alterations when associated with this temperature rise in vivo. Thus, not using external sources to activate hydrogen peroxide would be a safer procedure to keep pulp tissue health.
A variety of light sources that greatly differ in their properties are available to be used for light activation of bleaching products. In this study, it was used a LED appliance with a power output of 500 mW/cm2 to promote the light activation of bleaching agents. Previous studies have shown some advantages of this type of light source, such as less temperature increase in the pulp chamber (6,25). This can be explained by the emission of light by LEDs used with systems for light-activated bleaching, which is within the blue range and does not extend as far into the infrared spectral range as QHT or plasma arc lamps do. However, thermal pulp damage from LED-systems cannot be absolutely excluded and has to be considered especially when more powerful LEDs are used for a longer period of time (5).
It is still questionable whether the light activation results in better tooth whitening in comparison with non-activated bleaching procedures. So, the application of light should be done carefully and following the manufacturer's recommendations in order to avoid undesired pulp responses.
References
1. Goldstein RE. In-office bleaching: where we came from, where we are today. J Am Dent Assoc 1997;128(Suppl):11S-15S. [ Links ]
2. Miserendino L, Neiburger E, Pick R. Current status of laser in dentistry. Int Dent J 1987;56:254-7. [ Links ]
3. Sulieman M, Addy M, Rees JS. Surface and intra pulpal temperature rises during tooth bleaching: an in vitro study. Br Dent J 2005;199: 37-40. [ Links ]
4. Wetter NU, Walverde DA, Kato IT, Eduardo CP. Bleaching efficacy of bleaching gels activated by xenon lamp and 960-nm diode radiation. Photomed Laser Surg 2004;22:489-93. [ Links ]
5. Buchalla W, Attin T. External bleaching therapy with activation by heat, light or laser – A systematic review. Dent Mater 2007;23: 586-96. [ Links ]
6. Michida SMA, Passos SP, Marimoto ARK, Valera MC, Araujo MAM. Intrapulpal temperature variation during bleaching with various activation mechanisms. J Appl Oral Sci 2009;17:436-9. [ Links ]
7. Benetti AR, Valera MC, Mancini MN, Miranda CB, Balducci I. In vitro penetration of bleaching agents into the pulp chamber. Int Endod J 2004; 37:120-4. [ Links ]
8. Hanks CT, Fat JC, Wataha JC, Corcoran JF. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials in vitro. J Dent Res 1993;72:931-8. [ Links ]
9. Leonard RH, Bentley C, Eagle JC, Garland GE, Knight MC, Phillips C. Nightguard vital bleaching: a long-term study on efficacy, shade retention, side effects, and patients' perceptions. J Esthet Restor Dent 2001;13:357-69. [ Links ]
10. Bränström M. Dentinal and pulpal response III: Application of an air stream to exposed dentin. Long observation period. Acta Odontol Scand 1960;18:235-52. [ Links ]
11. Bränström M. Dentinal and pulpal response IV: Some experiments with heat and pressure illustrating the movement of odontoblasts into the dentinal tubules. Oral Surg Oral Med Oral Pathol 1962;15:203-12. [ Links ]
12. Goodis HE, White JM, Andrews J, Watanabe LG. Measurement of temperature generated by visible light cure lamps: an in vitro model. Dent Mater 1998;5:230-4. [ Links ]
13. Tjan AHL, Dunn JR. Temperature rise produced by various light generations through dentin barriers. J Prosthet Dent 1998;59: 433-8. [ Links ]
14. Guimarães JGA. Analise térmica da câmara pulpar em dentes humanos submetidos ao clareamento com laser diodo – estudo in vitro [thesis]. São Paulo (SP): Faculdade de Odontologia da USP; 2004. [ Links ]
15. Glickman GN, Frys H, Baker FL. Adverse response to vital bleaching. J Endodont 1992;18:351-4. [ Links ]
16. Bowles WH, Thompson LR. Vital bleaching: the effects of heat and hydrogen peroxide on pulpal enzymes. J Endodont 1986;12: 108-12. [ Links ]
17. Greenwall L. Bleaching techniques in restorative dentistry. London (UK): Martin Dunitz; 2001. [ Links ]
18. Zach L, Cohen G. Pulp response to externally applied heat. Oral Surg 1965;19:515-30. [ Links ]
19. Hein DK, Ploeger BJ, Hartup JK, Wagstaff RS, Palmer TM, Hansen LD. In-office vital tooth bleaching – what do lights add? Compend Contin Educ Dent 2003;24:340-52. [ Links ]
20. Clinical Research Associates. New generation on in-office vital tooth bleach, Part 2. CRA Newsletter 2003;27:1-2. [ Links ]
21. Papathanasiou A, Kastali S, Perry RD, Kugel G. Clinical evaluation of a 35% hydrogen peroxide in-office whitening system. Compend Contin Educ Dent 2002;23:335-48. [ Links ]
22. Leonard RH, Sharma A, Haywood VB. Use of different concentrations of carbamide peroxide for bleaching teeth: an in vitro study. Quintessence Int 1998;29:503-7. [ Links ]
23. Zanin F, Brugnera Junior A, Campos DHS, Zanin VOZ. Clareamento dental com Laser e LED. RGO 2003;51:143-6. [ Links ]
24. Nathanson D. Vital tooth bleaching: Sensitivity and pulpal considerations. J Am Dent Assoc 1997;128(Suppl):41S-44S. [ Links ]
25. Carrasco TG, Carrasco-Guerisoli LD, Fröner IC. In vitro study of the pulp chamber temperature rise during light-activated bleaching. J Appl Oral Sci 2008;16:355-9. [ Links ]
 Correspondence:
Correspondence:
Rua Jose Ferreira, 92, Jd Aquarius
Sao Jose dos Campos, SP – Brazil
12246-004.
E-mail: femollica@gmail.com
Received: January 23, 2010
Accepted: September 26, 2010
Conflict of Interest Statement: The authors state that there are no financial and personal conflicts of interest that could have inappropriately influenced their work.













