Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.58 no.1 Porto Alegre Jan./Mar. 2010
ORIGINAL ORIGINAL
Parabens do not increase fluoride uptake by demineralized dental enamel
Parabenos não aumentam incorporação de fluoreto pelo esmalte dental desmineralizado
Vanessa Silva Tramontino; Daniela Labbate; Cínthia Pereira Machado Tabchoury1; Jaime Aparecido Cury
Universidade Estadual de Campinas, Faculdade de Odontologia, Departamento de Ciências Fisiológicas. Av. Limeira, 901, Caixa Postal 52, 13414- 903, Piracicaba, SP, Brazil
ABSTRACT
OBJECTIVE: To evaluate whether methylparaben and propylparaben, which present a similar chemical structure, increase fluoride uptake by demineralized dental enamel when present in buffered solutions.
METHODS: The study comprised an in vitro experiment using blocks of bovine dental enamel with artificial carious lesions. Enamel blocks were exposed to the following treatment (n=12): fluoride solution (200 ppm fluoride) - control; solution containing fluoride and 13 mM methylparaben; solution containing fluoride and 13 mM propylparaben in 35% propylene glycol; solution containing fluoride in 35% propylene glycol. All solutions were buffered (0.01 M cacodilate) and the pH was adjusted to 6.27. The blocks were exposed to the treatment solutions in the proportion of 2 ml per mm2 of exposed enamel area and fluoride formed was estimated after removing an enamel layer by acid etching. Fluoride extracted was determined by ion specific electrode and the amount of enamel removed was estimated by phosphorus analysis. ANOVA followed by Tukey's test were used for statistical analysis, with significance level at 5%.
RESULTS: The dental blocks of treatment groups containing both parabens and the control group presented similar fluoride concentration in enamel and no statistical difference was observed among them (p>0.05). The dental blocks of treatment group containing fluoride and propylene glycol showed the lowest value of fluoride present in enamel, which was significantly different from the control and fluoride and methylparaben groups (p<0.05).
CONCLUSION: Methyl and propylparaben in a buffered solution do not enhance fluoride uptake by demineralized dental enamel.
Indexing terms: dental caries; dental enamel; fluorides.
RESUMO
OBJETIVO: Avaliar se o metilparabeno e o propilparabeno, os quais apresentam uma estrutura química similar, aumentam a incorporação de fluoreto pelo esmalte dental desmineralizado quando presentes em soluções tamponadas.
MÉTODOS: O estudo envolveu um ensaio in vitro, com 48 blocos de esmalte dental bovino com lesão cariosa artificial. Os blocos de esmalte, 12 para cada grupo, foram expostos aos seguintes tratamentos: grupo 1 (controle), solução de fluoreto (200 ppm F), Grupo 2, solução contendo fluoreto e metilparabeno 13 mM; Grupo 3 solução contendo fluoreto e propilparabeno 13 mM em propilenoglicol 35% e Grupo 4, solução contendo fluoreto em propilenoglicol 35%. Todas as soluções foram tamponadas (cacodilato 0,01 M) e o pH ajustado para 6,27. Os blocos foram expostos a soluções de tratamento na proporção de 2 ml por mm2 da área de esmalte exposta e fluoreto formado foi estimado após remoção de uma camada de esmalte por ataque ácido. Flúor extraído foi determinado por eletrodo específico e a quantidade de esmalte removido foi estimada pela análise de fósforo. Análise de variância seguida do teste de Tukey foi usada para análise estatística, com nível de significância de 5%.
RESULTADOS: Os blocos dentais dos grupos de tratamento contendo ambos parabenos e do grupo controle apresentaram concentrações de flúor similar no esmalte e nenhuma diferença estatística entre eles foi observada (p>0,05). Os blocos dentais do grupo de tratamento contendo fluoreto e propilenoglicol mostraram o valor mais baixo de flúor presente no esmalte, o qual foi significantemente diferente daquele dos grupos controle e flúor e metilparabeno (p<0,05).
CONCLUSÃO: Metilparabeno e propilparabeno em uma solução tamponada não aumentam a incorporação de flúor pelo esmalte dental desmineralizado.
Termos de indexação: cárie dentária; esmalte dentário; fluoretos.
INTRODUCTION
The decline in dental caries has been explained by the widespread use of fluoride1-2, which prevents and controls this disease3-4 by decreasing dental demineralization and increasing remineralization5-6. Fluoride can be delivered by several systems7, and among these, mouthrinses are recommended for community programs or self application8. In addition, these products have been suggested for patients at high caries risk9 and for prevention of root caries10.
Fluoride reactivity with dental enamel may be modified by the solution pH11-13 or interaction with other components of the formulation, such as antibacterial agents14 or detergents15-16. On the other hand, a recent study reported that some mouthrinse formulations showed an increase in fluoride reactivity with dental enamel17 and the preservative methylparaben seemed to be responsible for this effect18. However, it was also observed that the treatment solution containing methylparaben showed the lowest pH, around 5.6. Furthermore, apart from methylparaben, other parabens, such as propylparaben are also used as preservatives in hygiene products19.
Thus, the purpose in the present study was to evaluate whether methylparaben and propylparaben, which present a similar chemical structure, increase fluoride uptake by demineralized dental enamel when present in a buffered fluoridated solution.
METHODS
Experimental design
Forty-eight blocks of dental enamel were obtained from bovine incisor teeth and caries-like lesions were induced. They were randomly divided into 4 experimental groups (n=12) and exposed to fluoridated solutions, containing 200 ppm of fluoride. The experimental groups were: Group 1) fluoride - control; Group 2) fluoride and 13 mM methylparaben; Group 3) fluoride and 13 mM propylparaben in 35% propylene glycol; Group 4) fluoride in 35% propylene glycol. Propylene glycol was necessary to solubilize propylparaben due to its low hydrosolubility20. The fluoride concentration utilized in this study was 200 ppm instead of the usual 226 ppm, because according to pilot studies, a higher concentration of propylene glycol would be necessary to solubilize 226 ppm of fluoride. All solutions in this experiment were buffered (0.01 M cacodilate) and pH was adjusted to 6.27, which is the pKa of cacodilate21, the buffer chosen because it presents a pKa value next to the pH of an aqueous solution containing only NaF, which is around 6.117-18. Group 4 was proposed to evaluate any influence of propylene glycol on fluoride reaction with enamel. Fluoride concentration was confirmed in the treatment solutions before the exposure to the dental blocks. The time of exposure was 10 minutes under agitation and 2 ml of treatment solution was used for each mm2 of exposed dental block area. The pH of the treatment solutions was determined after reaction with dental blocks, and fluoride uptake by enamel was quantified. A biopsy was performed by removing a layer of enamel from each dental block by means of acid etching. The fluoride and phosphorous content in the extracts were determined.
Fluoride determination in treatment solutions
Fluoride concentration in the solutions was determined after buffering 1:1 with TISAB II (1.0 M acetate buffer pH 5.0, containing 1.0 M NaCl and 0.4% CDTA). The analyses were made in duplicate using a specific electrode ORION 96-06 and a previously calibrated ion analyzer EA 940 (Orion, Boston, USA).
pH determination
The pH of the solutions was determined after the reactivity test, using a glass electrode and a pHmeter (Procyon, São Paulo, Brazil) calibrated with standard buffers pH 4.0 and 7.0 (Orion, Beverly, USA).
Preparation of the dental blocks and induction of caries-like lesions
Blocks of dental enamel (4 x 4 x 2 mm) were obtained from sound bovine incisor teeth that had been sterilized by storage in a 2% formaldehyde (Chemco, Campinas, Brazil) solution (pH 7.0) at room temperature for at least 30 days22-23. The dentin was flattened, and the enamel surface was flattened and polished with 400, 600, 1,200 grit Al2O3 abrasive papers and polishing cloths with 1 µm diamond paste, respectively. During these procedures, the dental blocks were moistened with distilled and deionized water to avoid cracks in enamel. After this, the blocks were measured with a digital pachymeter (Mitutoyo, Suzano, Brazil) to determine the exposed enamel area (mm2), which was around 16 mm2 per block. The surfaces of all the blocks, except the enamel surface, were protected with a layer of acid-resistant varnish. Artificial carious lesions were produced in all dental blocks by immersion in a solution containing 0.74 mM phosphorous, 1.28 mM calcium, 50 mM acetate buffer, pH 5.0, 0.03 µg F/ml at 37ºC24, in a proportion of 2 ml solution/mm2 of exposed enamel, for 16 h, after which, all dental blocks were stored in a refrigerated and humid environment (4ºC).
Reactivity of treatment solutions with dental enamel
The dental blocks were immersed in the treatment solutions (proportion of 2 ml solution/mm2 of enamel surface) at room temperature and under slow agitation. After 10 min, the blocks were washed for 1 min with distilled and deionized water and stored as previously described.
Determination of fluoride in dental enamel
After exposure to treatment solutions, an enamel layer was removed from all dental blocks by acid immersion in 0.5 ml of 0.5 M HCl under agitation for 30 sec, followed by buffering with the same volume of TISAB II pH 5.0 modified with 20 g of NaOH/L25-26. Fluoride concentration in the extracts was determined using an ion analyzer ORION EA 940 and an ion specific electrode ORION 96-0917, previously calibrated with standards of 0.02 to 1.28 µg F/ml (Orion, Beverly, USA). The quantity of enamel (gram) removed in the acid attack was determined by measuring inorganic phosphorous (Pi) by a colorimetric method27, using spectrophotometer at 660 nm. An enamel density value of 2.92 was considered in order to calculate the amount of enamel that was removed28. The results were expressed as mg F/g in enamel.
Statistical analysis
Fluoride data in the enamel layer were submitted to ANOVA followed by Tukey's test. For these analyses, SAS software was used and significance limit was established at 5%.
RESULTS
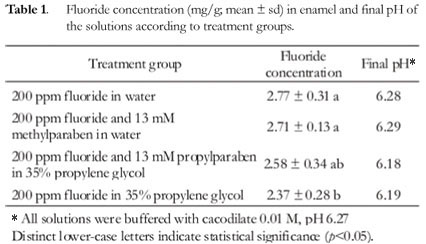
Table 1 shows the concentration of fluoride in enamel and the pH of the treatment solutions after reaction with the dental blocks. The dental blocks of treatment groups containing both parabens and the control group presented similar fluoride concentration in enamel and no statistical difference was observed among them (p>0.05). The dental blocks of the treatment group containing fluoride and propylene glycol showed the lowest value of fluoride present in enamel, which differed significantly from the control and fluoride and methylparaben groups (p<0.05). The treatment group containing fluoride and propylparaben did not differ significantly from the propylene glycol group with regard to fluoride present in enamel (p>0.05). The final pH of the control, fluoride and methylparaben treatment solutions practically did not differ from the initial pH, however, in the treatment solutions containing propylparaben and propylene glycol there was a decrease of around 0.1 unit in the final pH.
DISCUSSION
The results obtained in this study bring new information to the observation reported by Tabchoury et al.17 and Arthur et al.18, who reported higher fluoride uptake by demineralized enamel in the presence of methylparaben. The present findings suggest that the effect reported by these previous studies may be due to the pH of methylparaben solution, which was 5.618. It is well known that the pH plays a fundamental role in fluoride uptake by dental enamel5,11,13 and its lowering from 7 to 5.5 implies a 4 times higher fluoride incorporation by the substrate12. Thus, as a buffer was used in the present study to maintain the pH of all treatment solutions at 6.27, no significant difference could be observed between the control group and the group containing methylparaben with regard to fluoride incorporation by enamel, suggesting that methylparaben does not affect this process of fluoride incorporation by enamel. Furthermore, the present study suggests that fluoride uptake by enamel may be influenced by the presence of a buffering system and further studies should be conducted to better evaluate this effect.
Propylparaben was used in another treatment group due to its chemical similarity to methylparaben. If methylparaben presented some effect due to its chemical properties, propylparaben would probably demonstrate the same effect. Nevertheless, propylparaben did not interfere with fluoride uptake by dental enamel, supporting the observation that the pH of the solution was responsible for the effect of methylparaben observed in other studies17-18.
Another important observation in this study was that the fluoridated solution that contained propylene glycol presented a lower fluoride uptake by enamel than the control group. Propylene glycol was used to solubilize propylparaben because of its hydrophobic characteristic, which may be responsible for the decrease in fluoride uptake. Other studies in the literature have reported interference of fluoride reactivity in dental enamel. Barkvoll et al.14, investigating the compatibility of chlorhexidine digluconate and sodium monofluorophosphate, verified that they are not clinically compatible, since a reaction occurs that results in the formation of an insoluble salt, which decreases fluoride uptake by enamel. Barkvoll15 and Franco & Cury16 evaluated the effect of solutions containing the anionic detergent sodium lauryl sulfate and verified lower fluoride uptake by enamel. However, in the present study the mechanism of this effect was not assessed.
CONCLUSION
Methyl and propylparaben in a buffered solution do not enhance fluoride uptake by demineralized dental enamel.
Acknowledgements
To Prof. Glaucia Maria Bovi Ambrosano, for her contribution in the statistical analysis. To Mrs. Mariza J. C. Soares and Mr. Waldomiro Vieira Filho, for their help with the laboratory analyses. To FAPESP, for the financial support (02/14064-6).
Collaborators
VS TRAMONTINO, D LABBATE, CPM TABCHOURY and JA CURY were responsible for the development of all the stages of this article.
REFERENCES
1. Clarkson JJ. International collaborative research on fluoride. J Dent Res. 2000;79(4):893-904. [ Links ]
2. Cury JA, Tenuta LMA, Ribeiro CCC, Paes Leme AF. The importance of fluoride dentifrices to the current dental caries prevalence in Brazil. Braz Dent J. 2004;15(3):167-74. [ Links ]
3. Brambilla E. Fluoride: is it capable of fighting old and new dental diseases? An overview of existing fluoride compounds and their clinical applications. Caries Res. 2001;35(Suppl 1):6-9. [ Links ]
4. Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004;38(3):182-91. [ Links ]
5. Larsen MJ. Chemical events during tooth dissolution. J Dent Res. 1990;69:575-80. [ Links ]
6. Featherstone J. The science and practice of caries prevention. J Am Dent Assoc. 2000;131(7):887-99. [ Links ]
7. Clarkson JJ, McLoughlin J. Role of fluoride in oral health promotion. Int Dent J. 2000;50(3):119-28. [ Links ]
8. Horowitz HS, Creighton WE, McClendon BJ. The effect on human dental caries of weekly oral rinsing with a sodium fluoride mouthwash: a final report. Arch Oral Biol. 1971;16(6):609-16. [ Links ]
9. FDI Comission. Mouthrinses and dental caries. Int Dent J. 2002;52(5):337-45. [ Links ]
10. Zimmer S. Caries: preventive effects of fluoride products when used in conjunction with fluoride dentifrice. Caries Res. 2001;35(Suppl 1):18-21. [ Links ]
11. Friberger P. The effect of pH upon fluoride uptake in intact enamel. Scand J Dent Res. 1975;83(6):339-4412. [ Links ]
12. Saxegaard E, Rölla G. Fluoride acquisition on and in human enamel during topical application in vitro. Scand J Dent Res. 1988;96(6):523-35. [ Links ]
13. Delbem AC, Cury JA. Effect of application time of APF and NaF gels on microhardness and fluoride uptake of in vitro enamel caries. Am J Dent. 2002;15(3):169-72. [ Links ]
14. Barkvoll P, Rölla G, Bellagamba S. Interaction between chlorhexidine digluconate and sodium monofluorophosphate in vitro. Scan J Dent Res. 1988;96(1):30-3. [ Links ]
15. Barkvoll P. Effect of sodium lauryl sulfate on the uptake of fluoride from NaF and MFP by etched enamel in vitro. J Biol Buccale. 1991;19(3):235-9. [ Links ]
16. Franco EM, Cury JA. Effect of Plax prebrushing rinse on enamel fluoride deposition. Am J Dent. 1994;7(2):119-21. [ Links ]
17. Tabchoury CPM, Pierobon CN, Cury JA. Concentration and bioavailability of fluoride in mouthrinses prepared in dispensing pharmacies. J Appl Oral Sci. 2005;13(1):41-6. [ Links ]
18. Arthur RA, Tabchoury CPM, Giancristófaro M, Del Bel Cury A, Cury JA. Effect of preservatives on reactivity of fluoride with dental enamel. RGO - Rev Gaúcha Odontol. 2007;55(4):375-80. [ Links ]
19. Pinto TJA, Kaneko TM, Ohara MT. Controle biológico de qualidade de produtos farmacêuticos, correlatos e cosméticos. São Paulo: Atheneu; 2000. [ Links ]
20. Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol. 2002;40(10):1335-73. [ Links ]
21. Dawson RM, Elliot DC, Elliot WH, Jones KM. Data for biochemical research. 3rd edition. New York: Oxford Science Publications; 1995. [ Links ]
22. White DJ. Reactivity of fluoride dentifrices with artificial caries. Caries Res. 1987;21(2):126-40. [ Links ]
23. Cury JA, Rebello MAB, Del Bel Cury AA, Derbshyre MTVC, Tabchoury CPM. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000;34(6):491-7. [ Links ]
24. Queiroz CS, Hara AT, Paes Leme AF, Cury JA. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz Dent J. 2008;19(1):21-7. [ Links ]
25. Paes Leme AF, Tenuta LMA, Del Bel Cury AA, Tabchoury CPM, Cury JA. Efeito da associação da aplicação de fluoreto profissional e uso de dentifrício no esmalte dental. RGO - Rev Gaúcha Odontol. 2007;55(1):35-40. [ Links ]
26. Maia LC, Souza IP, Cury JA. Effect of a combination of fluoride dentifrice and varnish on enamel surface rehardening and fluoride uptake in vitro. Eur J Oral Sci. 2003;111(1):68-72. [ Links ]
27. Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66: 375-400. [ Links ]
28. Lazzari EP. Dental Biochemistry. 2 ed. London: Henry Kimpton Publishers; 1976. [ Links ]
Received on: 27/2/2009
Final version resubmitted on: 30/7/2009
Approved on: 9/10/2009
1 Correspondence to: CPM TABCHOURY. E-mail: <cinthia@fop.unicamp.br>













