Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.59 no.3 Porto Alegre Jul./Set. 2011
ARTIGO ORIGINAL / ORIGINAL ARTICLE
Shear bond strength and etching pattern of self-etching bonding agents on ground and intact enamel
Resistência ao cisalhamento e padrão de condicionamento de sistemas adesivos autocondicionantes em esmalte intacto e preparado
Jean Carlos Nogueira ARAÚJOI; Mário José Pinto LOPESI Gabriela Queiroz de Melo MONTEIROII. IV; Carlos Magno dos ANJOSII; Marcos Antonio Japiassú Resende MONTESIV
I Universidade de Pernambuco, Faculdade de Odontologia. Recife, PE, Brasil
IIUniversidade Federal de Pernambuco, Departamento de Física, Recife, PE, Brasil
IIIPrivate Dental Practioner. Rio de Janeiro, RJ, Brazil
IVUniversidade de Pernambuco, Faculdade de Odontologia, Departamento de Odontologia Restauradora
ABSTRACT
Objective
The aim of this study was to evaluate the shear bond strength of four self-etching adhesive systems on intact and ground enamel, and also to evaluate the morphology of the enamel surface after etching.
Methods
A total of 100 bovine central lower incisors were randomly divided into five groups: Single Bond (3M ESPE, St. Paul, USA), Adper Prompt L Pop (3M ESPE, St. Paul, USA), Clearfil SE Bond (Kuraray America, USA), One-up Bond F (Tokuyama Corp., Shibuya-ku, Tokyo, Japan) and AdheSE (Ivoclar/Vivadent, Schaan, Liechtenstein). Each group was subdivided (n=10), according to the surface preparation (intact or ground enamel). For intact enamel, the teeth were pumiced and ground enamel surfaces were obtained with wet 320-grit SiC paper. A circular (4mm) bonding area was demarcated and resin rods (Filtek Z250, 3M ESPE, St. Paul, USA) were built (5mm) for the shear test, followed by failure mode analysis using Scanning Electron Microscopy. In addition, 12 teeth were prepared for the evaluation of the etching pattern by Scanning Electron Microscopy. Statistical analysis of the data was performed with two-way ANOVA and Tukey's test (p<0.05).
Results
Significant differences were observed between the Shear bond strength values for the adhesive systems (p<0.001). No differences were found between the two substrates (p=0.598) nor any interaction between the adhesive systems versus substrate (p=0.404). The etching patterns were generally observed as mild when compared to phosphoric acid, except for Adper Prompt L Pop (3M ESPE, St. Paul, USA), which was similar to phosphoric acid.
Conclusion
Shear bond strength was not influenced by the type of substrate (intact or ground enamel), and no correlation was observed between the Shear bond strength values and the etching pattern of the self-etching adhesives studied.
Termos de indexação: Dentin-bonding agents. Dental enamel. Shear strength.
RESUMO
Objetivo
Avaliar a resistência ao cisalhamento e o padrão de condicionamento de quatro sistemas adesivos autocondicionantes em esmalte intacto e preparado.
Métodos
Cem incisivos centrais inferiores bovinos foram aleatoriamente divididos em cinco grupos: Single Bond (3M ESPE, St. Paul, USA), Adper Prompt L Pop (3M ESPE, St. Paul, USA), Clearfil SE Bond (Kuraray America, USA), One-up Bond F (Tokuyama Corp., Shibuya-ku, Tokio, Japan) e AdheSE (Ivoclar/Vivadent, Schaan, Liechtenstein). Cada grupo foi subdividido (n=10) de acordo com o tipo de superfície. Os dentes com esmalte intacto foram limpos com pasta de pedra pommes/água e os com esmalte preparado tiveram a superfície do esmalte desgastada com lixas d'água (n.320). Uma área circular (4mm) foi demarcada para adesão e os cilindros de resina (Filtek Z250, 3M ESPE, St. Paul, USA) foram confeccionados (5mm). Após o ensaio resistência ao cisalhamento, foi realizada a avaliação do modo de fratura. Doze dentes foram preparados para avaliação do padrão de condicionamento em Microscopia Eletrônica de Varredura. A análise estatística dos dados foi realizada através do teste ANOVA para 2 fatores e teste de Tukey (p<0.05).
Resultados
Diferenças estatisticamente significante foram observadas para a resistência ao cisalhamento entre os sistemas adesivos (p<0.001). Não foram observadas diferenças entre os substratos (p=0.598) ou ainda interações entre o sistema adesivo versus o substrato (p=0.404). Os padrões de condicionamento observados foram classificados em leve em relação ao padrão após o condicionamento com o ácido fosfórico, com exceção do padrão observado para o adesivo Adper Prompt L Pop (3M ESPE, St. Paul, USA).
Conclusão
A resistência ao cisalhamento não foi influenciada pelo tipo de substrato, e, nenhuma correlação entre os valores de resistência ao cisalhamento e padrão de condicionamento dos adesivos autocondicionantes estudados foi observado.
Termos de indexação: Adesivos dentinários. Esmalte dentário. Resistência ao cisalhamento.
INTRODUCTION
The bonding of resins to the enamel surface, as established by Buonocore in 1955, is to this day considered a highly reliable routine procedure in dental practice. The ability of adhesive resins to penetrate through the microporosities created on the enamel surface by acid etching was initially described by Buonocore et al.1 and was attributed to an increase in the area of contact and surface energy, due to the partial dissolution of enamel prisms and posterior resin infiltration and polymerization in situ. Clinically, the micro-leakage responsible for secondary caries at the tooth-restoration interface and post-operative sensitivity has been partially overcome in cavities with a cave-surface angle in the enamel.
While the clinical procedures of enamel acidetching have changed the way in which esthetic restorative dentistry is practiced, researchers continue to search for and develop materials and techniques that allow resins (hydrophobic materials) to bond to the dynamic dentin substrate2. Indeed, bonding to dentin was not as easily achieved as it was for enamel which could be explained by its high organic content, intrinsic wetness and the presence of the smear layer; the initial bonding systems, therefore, did not perform well on dentin3. In fact, the removal of the smear layer by acid-etching increased the outward flow of dentinal fluid, contributing to the occurrence of adhesive failure. The solution to this problem was the development of bonding systems with primers containing hydrophilic and hydrophobic functional groups, allowing resin infiltration through the wet, demineralized dentin substrate, creating a resin-dentin interdiffusion zone called the hybrid layer4-5.
This bonding procedure, termed the total-etch technique, uses 10-40% phosphoric acid solutions to demineralize enamel and dentin simultaneously; the capacity of the ionic dissociation of this acid has been observed to cause an excessively deep demineralization in the dentin substrate, exposing the collagen fiber network to a depth that was not easily filled by the adhesive resins, leaving the collagen fibers exposed in the deepest parts of the dentin, and subjecting them to hydrolysis and degradation, compromising the restoration over time6. In order to improve the durability and reduce the sensitivity of the total-etch technique, a new bonding agent category was developed. The so-called self-etching bonding agents contain acid monomers that etch and infiltrate simultaneously in enamel and dentin7. Thus, the application procedures involving the self-etching bonding agents have been regarded as an easy and reliable alternative towards achieving the optimal conditioned surface for bonding8.
However, controversy surrounds the evolution of the bonding agents towards a better adhesion on the dentinal substrate and the development of self-etching systems, while incorporating enamel bonding procedures in these systems. There is a concern that manufacturers sacrifice the stability of the enamel bond while trying to simplify and achieve a reliable, improved dentin bond. The most evident challenges of these systems relate to their etching capacity and strength of the bond to enamel, due to the difference in their acidity and demineralization depths, which are considered to be milder and less retentive than phosphoric acid9, and to the stability of these highly hydrophilic formulations over time10.
It has been suggested that, when using self-etching systems, the strength of the bond to enamel is lower and not sufficient to seal the restoration when compared to dentin3. Furthermore, even lower strengths of the bond to intact enamel surfaces have been reported, which may be related to the chemical and micro-morphological differences between ground and intact enamel11-12.
Thus, this study aimed to measure and evaluate the SBS of self-etching bonding systems on intact and ground enamel and, in addition, to analyze and correlate the surface morphology with SBS values after etching and bonding with these systems.
METHODS
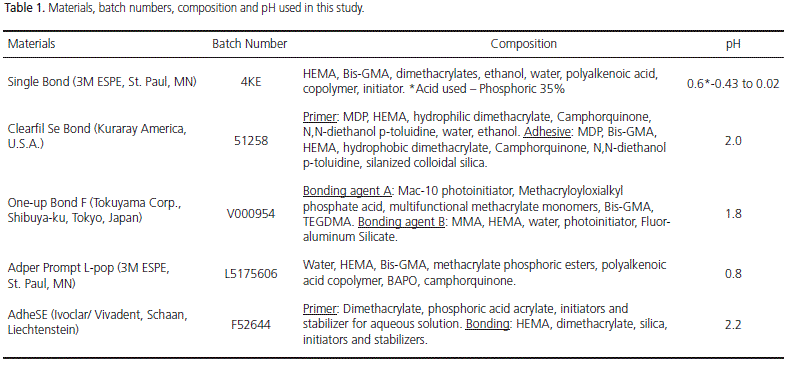
The materials, manufacturers, composition, pH and batch numbers used in this study are listed in Table 1. Freshly extracted lower bovine central incisors (n=112) obtained from a local abattoir were disinfected with 0.5% chloramine for 1 hour and placed in frozen storage for as much as one month. Incisors were used to evaluate SBS (n=100) and surface etching patterns (n=12). The teeth had their roots sectioned 2 mm below the cementumenamel junction with a low-speed diamond disk under water, and then embedded with a self-curing acrylic resin in PVC cylinders (20 X 20 mm), with the buccal surfaces projected 1 mm outward from the cylinder's edge.
For SBS testing, 100 teeth were divided into two groups for substrate preparation on intact and ground enamel (50 teeth each). For the ground enamel group, a flat surface was obtained on a grinding wheel using a 320- grit SiC under water. For the intact enamel group, the flat incisal third of the crown, present in central bovine lower incisors, were pumiced before being used. The bonding area was demarcated by placing adhesive tape on the prepared surface, in which a 4 mm diameter hole had been punched.
The specimens were randomly assigned to 5 groups of 20 specimens each, in accordance with the bonding system applied, and each group was subdivided into 2 subgroups (10 for intact and 10 for ground enamel surfaces) as follows: Group 1 (control group): Single Bond (3M ESPE, St. Paul, MN) (SB); Group 2: Clearfil SE Bond (Kuraray America, U.S.A.) (SEB); Group 3: Adper Prompt L-Pop (3M ESPE, St. Paul, MN) (POP); Group 4: One-up Bond F (Tokuyama Corp., Shibuyaku, Tokyo, Japan) (OUB); Group 5: AdheSE (Ivoclar/ Vivadent, Schaan, Liechtenstein) (ADH). The adhesive systems were applied according to the manufacturers' recommendations, as follows:
- Group 1 (SB): The surface was acid-etched with 35% phosphoric acid (15s); rinsed with running water (20s); excess water was removed with tissue paper; adhesive application in two layers with a disposable brush; gently air-dried with oil-free compressed air (5s); lightcured (10s).
- Group 2 (SEB): Primer application (20s); dried with a mild air flow, the same as for group 1; adhesive application; gentle air flow; light-curing (10s).
- Group 3 (POP): Primer/ adhesive application; gently air dried, as for groups 1 and 2; light-cured (10 s).
- Group 4 (OUB): Primer/ adhesive application; left undisturbed (20s); gently air dried, as for groups 1, 2 and 3; light-cured (10s).
- Group 5 (ADH): Primer application (20s); gently air dried (5s); adhesive application; gently air dried (5s); light-cured (10s).
A 5mm high composite rod (Filtek Z250, 3M ESPE, St. Paul, MN, USA) was built on the bonding areas, with the composite applied in three increments using a silicone mold, with each increment polymerized for 30s. All light-curing procedures used an Ultralux curing unit (Dabi Atlante, Ribeirão Preto, Brazil) with a light intensity of 500mW/cm². The light intensity was measured with a radiometer (Curing radiometer, model 100, Demetron/ Kerr, Danbury, CT 06810, USA). The specimens were stored in distilled water at 37°C for 24 hours and then submitted to a SBS test in a Kratos Universal Testing Machine, model K2000MP (Taboão da Serra, Brazil), with a cross-head speed of 0.5mm/min. SBS was performed using a 0.5mm metallic steel tape which formed a loop that completely enclosed the composite rod13.
After testing, SBS values were recorded, each specimen was gold-sputter-coated (Balzers - SCD 050 Sputter Coater, Liechtenstein) and observed by SEM (JEOL, JSM 5200, Scanning Electron Microscope, Tokyo, Japan) for an evaluation of the fracture pattern. Fracture modes were classified into one of three types: a) type 1 - adhesive
failure between the adhesive resin and enamel, and partial cohesive failure in adhesive resin; b) type 2 - partial cohesive failure in enamel; c) type 3 - cohesive failure in composite resin.
The SBS results were submitted to ANOVA variance analysis (p<0.05) and to multiple comparisons using Tukey's Test (p<0.05). Statistical analysis was carried out using the SPSS software system.
Additionally, the pH values for each bonding system were digitally measured with a digital pH-meter (pHmetro Digimed DM-20 μP, Digicrom, São Paulo, Brazil), in accordance with the following protocol: dilution of 2ml of the etching agent in 3ml of 70% ethanol, without stirring, at room temperature (20-25°C). The first measurement was performed after a 5min interval, followed by two other measurements at 1 minute intervals. The pH values were the result of the arithmetic means of these three results, and are shown in Table 1.
Etching pattern
Twelve teeth were used for this evaluation, and were submitted to the same surface preparation as for the bonding procedures. Six teeth were ground with 320-grit SiC under water in order to obtain a flat grounded surface with standardized smear layers. Incisal and radicular portions of the crowns were removed to obtain rectangular specimens. The other six teeth were simply pumiced. Ten teeth were assigned to the 5 groups, one being intact and the other ground, for each group. Two teeth remained as controls. For all the groups, the etching agents were applied as previously described and dehydrated in ascending levels of acetone: 50%, 75% and 100%, for 10 min. in each concentration, in an ultrasonic bath, in order to dissolve the residual monomers in enamel. Afterwards, the specimens were stored at 60°C for 1 hour, gold-sputter-coated and observed by SEM.
Statistical analysis
Statistical analysis was carried out using SPSS software 11.0 (Statistical Package for the Social Sciences, Chicago, USA). Means and standard deviations were calculated. Normal distributions were tested by the Shapiro- Wilk test. Two-way ANOVA was calculated to see if there were any differences between the groups and Levene's statistic was used to test for homogeneity of variances. In those cases with significant differences, the post-hoc Tukey test was used. Statistical significance for all tests was assumed to be p<0.05.
RESULTS
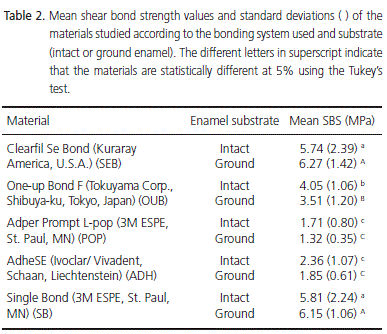
The SBS results, according to the bonding agent used and the type of substrate (intact or ground enamel), are shown in Table 2. The highest SBS values were obtained by SEB and SB and the lowest values were obtained by POP followed by ADH. For SEB and SB, SBS was higher on the ground enamel surface and the opposite occurred with the other three systems (ADH, POP and OUB). Twoway ANOVA demonstrated that there were significant differences between the bonding agents (p<0.001) although no significant differences were found between the substrates (p=0.598); in addition, no significant interaction was found between the bonding agent and the type of substrate (p=0.404). Only one specimen of SEB on ground enamel surface showed a Type 2 failure that was cohesive in enamel. All the other specimens, regardless of the adhesive or substrate, presented Type 1 adhesive failure.
Etching pattern
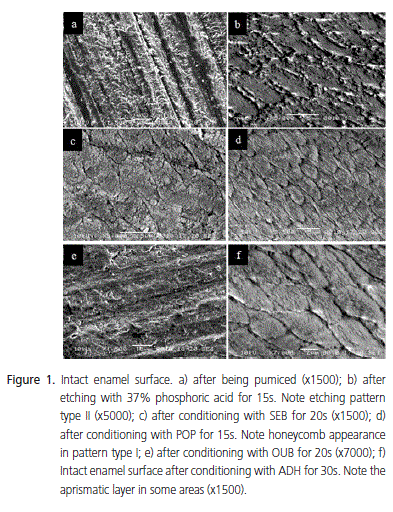
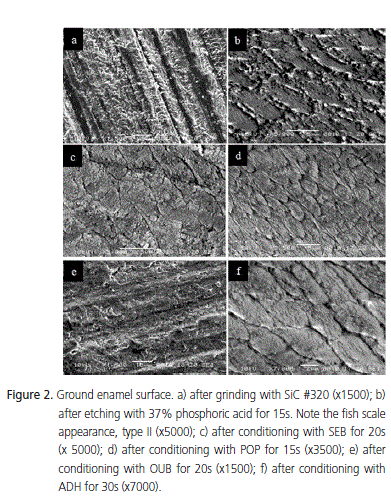
A very smooth surface was found with intact enamel (Figure 1a). Thus, conditioning with 37% phosphoric acid for 15s completely removed the aprismatic layer, exhibiting many porosities and a great number of enamel crystallites. At higher magnification, a type II etching pattern was observed, with the individualization of the enamel rods (Figure 1b). Conversely, self-etching agents were not capable of completely demineralizing the aprismatic layer of intact enamel (Figure 1c, 1e, 1f) with the exception of specimens treated with POP, which showed an etching pattern similar to that observed with 37% phosphoric acid, with good dissolution of the aprismatic layer and conditioning of the enamel rod cores (etching pattern type I - Figure 1d). ADH promoted a slight dissolution of the aprismatic enamel, mildly conditioning the underlying prisms in its core (etching pattern type I - Figure 1f). This was also observed for OUB, although in this case, the periphery of the prisms in the underlying layer was mildly conditioned (etching pattern type II - Figure 1e). SEB did not promote a good dissolution of the aprismatic layer, leaving it porous, conditioning neither the core nor the periphery of the underlying prisms (no etching pattern was observed - Figure 1c). The ground enamel surface without prior conditioning showed the presence of a smear layer, which covered the surface, making it impossible to view the enamel prisms (Figure 2a). After conditioning with 37% phosphoric acid for 15s, the smear layer was completely removed and the enamel surface showed a honeycomb aspect, with greater dissolution of the peripheral portion when compared to the core of the prisms, characterizing a type II etching pattern (Figure 2b). As for the selfetching systems, a well defined etching pattern could not be observed.
No clear morphological difference was observed on the enamel surface after conditioning with these systems. Even after the surface treatment, it was possible to see smear layer in some areas and also the grooves produced by SiC abrasive paper, making it difficult to view the enamel prisms. Again, surfaces treated with POP behaved differently, obtaining an etching pattern similar to that produced with 37% phosphoric acid (Figure 2c). ADH exhibited a slight dissolution of the smear layer with a mild etching pattern, also showing a discrete individualization of the enamel rods (Figure 2f). SEB also presented a mild etching pattern, characterized by the heterogeneous dissolution of the prismatic and interprismatic region, with a discrete dissolution of the prisms (Figure 2d). OUB also presented a mild etching pattern although differentiation of the enamel prisms was almost impossible (Figure 2e).
DISCUSSION
Intact and ground enamel substrates used in this study are often present in clinical daily routine, therefore it is very important to determine whether these simplified bonding agents are as effective as conventional (total etch) systems when bonding to these substrates11.
Measuring bond strength on intact enamel involves some technical difficulties due to the convexity of this surface, which makes it difficult to obtain the flat surfaces needed for conventional tests. Using bovine lower incisors makes it possible to obtain a flat enamel surface without the need for microtensile or microshear bond tests. Many similarities are found between human and bovine enamel: crystal orientation, prism dimensions, chemical composition of the protein matrix, percentage calcium, carbonate content and hardness14-15. It has also been reported that the demineralization pattern of different acids such as phosphoric, maleic and citric acids, are similar between human and bovine enamel9. In addition, previous reports state that histological and adhesive properties of bovine and human enamel are similar, enabling them to be valuable substitutes for bonding tests. Advantages in relation to research ethics are also gained, since human samples are currently difficult to collect, and bovine teeth are easily found in large quantities16.
The SBS results found in this study were lower than those found in other studies; this may be explained by the type of loading used here. The influence of the different types of loading used for the shear bond tests was previously tested: orthodontic wire, chisel (recommended by ISO TR 11405, 1994) and metallic steel tape were used13. The highest results were obtained with the orthodontic wire (13.33MPa), followed by the chisel (7.81MPa) and then the metallic steel tape (4.87MPa); these values were dependent on the combination of tensions produced during the specimen testing. With the orthodontic wire, cohesive failures were mainly observed in the resin composite. When the chisel was used for loading, complex tensions were produced evolving into cleavage, tensile and compressive just before fracturing. The lowest shear bond strength values, obtained with the metallic steel tape, indicate that the tensions produced in this test are less complex, creating better conditions for the establishment of a stringent shear bond test, with most of the failures on the adhesive resin coming as a result of the high concentration of tangential forces. It was concluded that the metallic steel tape does not produce a point of support (fulcrum or momentum) in the resin rod, in addition, the point where the strength is applied does not vary.
Significant differences were found in the SBS values between the self-etching agents (p<0.05). The highest results were obtained for SB and SEB, for both intact and ground enamel (6.15 and 5.81MPa for SB; 6.27 and 5.74MPa for SEB, respectively). Although no significant difference was found between these agents, their etching patterns were completely different; SEB had a milder effect. Significantly lower SBS results for ground and for intact enamel were obtained with ADH and POP (1.85 and 2.36MPa; 1.32 and 1.71MPa, respectively), despite the fact that the etching pattern obtained with POP was very similar to the one obtained with phosphoric acid, in contrast to the etching pattern of ADH, which was much milder, no significant differences were found between them. Intermediate SBS results were obtained with OUB for ground and intact enamel (3.51 and 4.05MPa), these values were higher than those of POP and ADH, despite its mild etching pattern.
Additionally, no significant differences were found between the type of substrate, ground or intact (p>0.05). The demineralization produced by phosphoric acid (SB) was superior to all the self-etching systems, both on ground and intact enamel. It is interesting to note that, although the type of substrate did not influence SBS values, all selfetching bonding agents showed a more defined etching pattern on ground enamel.
It has been suggested that the dentin smear layer interferes with the bonding of self-etching agents in an inverse relationship to its thickness17. As for enamel, the conditioning of its surface with phosphoric acid promotes surface cleaning, greater surface roughness and energy. The conditioning of the enamel transforms a relatively smooth surface to an irregular surface with micro porosities that can be filled by a fluid resin or adhesive. The most acceptable mechanism to explain the adhesion to enamel is resin tag formation, which leads us to conclude that the quality of bonding to the enamel surface is strongly dependent on the irregularities formed on the surface and also on the capacity of the adhesive resin to penetrate the substrate. The introduction of self-etching bonding agents initiated a new way to condition dental substrates. In order to simplify the technique of bonding to dentin, the use of acidic substances that include resin monomers was proposed. These substances are applied simultaneously on to enamel and dentin and do not require a washing stage. In vitro studies show that etching patterns, produced by self-etching agents on enamel, were different to those produced by phosphoric acid, without compromising the quality of the bond strength. The bond strength in enamel was very similar to that obtained with conventional systems that require prior phosphoric acid etching18-19.
Conversely, some studies did not achieve acceptable bond strength when comparing self-etching to total etch systems, these low values being attributed to the lower acidity of self-etching systems, when compared to 37% phosphoric acid3,20. These results are in accordance with the results obtained in this study where, with the exception of SEB, all the other systems had significantly lower SBS values than SB, which employs a previous acid conditioning stage, using phosphoric acid. The etching pattern produced by phosphoric acid favors the formation of a strong mechanical interlocking of the resin on the conditioned surface, forming a stable bond. The total etch technique has been described as the procedure that is most recommended for adhesive restorations on enamel, being considered a safe and predictable procedure. The similar results obtained between SB and SEB can be explained by another possible bonding mechanism. It has been suggested that chemical bonding occurs between the hydroxyapatite of the enamel structure and the 10-MDP monomer present in SEB21.
The morphological structure of the intact enamel surface is different to the central portion, since it is deprived of prisms, is hypermineralized and contains more inorganic material. As the intact enamel is more mineralized, it contains more fluoride than ground enamel12. Moreover, a thick layer of aprismatic enamel can make the permeability of self-etching primers and bonding agents through the substrate difficult, leaving some areas partially unconditioned and resulting in a poor mechanical interlocking due to the deficient penetration of the adhesive resin into the micro-porosities of the intact enamel surface, thus lowering the bond strength. Despite the similarity between SBS in both substrates, the presence of this aprismatic layer could explain the milder etching pattern on the intact surface, compared to the ground enamel surface. Moreover, it seems logical to accept the fact that on smear layer-free surfaces, self-etching bonding agents have direct contact with the substrate (the smear layer would not represent a barrier to be surpassed), increasing its superficial roughness. This could explain the proximity of SBS results for intact and ground enamel17. In the present study, the formation of grooves was observed after pumicing, leaving the intact enamel surface with similar characteristics to the ground surface. The presence of grooves could be seen even after superficial treatment with the self-etching agents, in accordance with the findings of other authors11,22. This could also explain the absence of significant differences between the SBS in both substrates, where other factors such as surface energy variation may be responsible for the achievement of an adequate bonding on enamel19.
However, no correlation was found between bond strength values and the length of resin tags, even though bond strength values can be mainly attributed to the capacity of the resin to penetrate through the enamel prisms and crystallites12,23. The equilibrium between the depth of demineralization and the extension of monomer penetration is key to the formation of a high quality resin-enamel interface, which is the basic principle of selfetching adhesives24. Studies on the bonding mechanism of self-etching adhesives on enamel using TEM evaluations concluded that, even though the resin tags were relatively short, the nano-retention of these materials to the prismatic structure of the enamel could justify the bond strength values achieved, despite the different etching patterns25-26. This is in accordance with the results obtained in this study, where the similar SBS results between the total etch (SB) and the self-etch adhesive (SEB) did not correspond to the etching pattern obtained from SEM analysis.
The self-etching adhesives tested showed differences in the composition of the acid monomer and pH. This could explain the etching patterns obtained, which were dependent on the acidity of the adhesives and their aggressiveness in conditioning the enamel surface. The self-etching adhesive, SEB, is a two-step system with a relatively high pH (2.0) and a relatively low concentration of the acidic ester 10-MDP (25-30%) in its composition. The etching pattern obtained with SEB in this study did not present separate structures on the enamel surface, however precipitates were observed on the enamel surface, probably from residual 10-MDP and/or remainders of the dissolved enamel. This bonding agent had a milder effect on the intact enamel surface, in agreement with other authors22,27-29.
The other two-step, self-etching agent, ADH, contains phosphoric acid acrylates as conditioning agents, with a pH of 2.2. The obtained etching pattern was more evident on intact enamel, matching the SBS values. POP is a one-step system that contains a high concentration of phosphoric acid ester methacrylates (80%) and has a pH of between 0.8 and 1.0.26 This high acidity can be verified when observing the aggressive etching pattern on ground and intact enamel. However, the etching pattern of this agent did not show any correlation with its SBS values, which were amongst the lowest. The monomer present in its composition (2-metacryloyloxyethyl-dihydrogenephosphate) is responsible for the superficial dissolution of the enamel and subsequent formation of a threedimensional micro retentive pattern, similar to the one obtained by phosphoric acid. The other one-step system, OUB, is purchased in two bottles and has, as its acidic agent, MAC-10 with a pH of 1.8. The etching pattern obtained here was more evident on intact enamel, which could explain the SBS results on this substrate.
Acid-etching of the enamel surface produced substantial modifications, including the complete removal of the smear layer (ground enamel) and the aprismatic layer (intact enamel), resulting in micro-porosities, crystallite exposure and enamel prism individualization. The efficacy of the self-etching adhesives on intact enamel does not depend on their etching aggressiveness, despite differences in composition, making a correlation between monomer structure and bond strength very difficult. Considering that there was no correlation between bond strength and resin tag lengths, enamel bond strength appears to occur as a result of the resin's capacity to permeate the enamel prisms30.
CONCLUSION
Based on the results obtained from this study, it can be concluded that there were differences regarding the SBS values and surface etching pattern between the self-etching adhesives studied, no correlation was found between SBS and the morphology of the conditioned enamel surface, the type of substrate (intact or ground enamel) did not significantly influence the SBS values and there were no significant interactions between the selfetching agents and the type of substrate.
CollaboratorsJCN ARAÚJO was responsible for the shear strength test and the editing of the article. MJP LOPES was responsible for the shear strength test and for the editing of the article. GQM MONTEIRO and CM ANJOS were responsible for the Scanning Electron Microscopy and the editing of the article. MAJR MONTES was responsible for guidance and the editing of the article.
REFERÊNCIAS
1. Buonocore MG, Matsui A, Gwinnett AJ. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch Oral Biol. 1968;13(1):61-70. [ Links ]
2. Marshall SJ, Bayne SC, Baier R, Tomsia AP, Marshall GW. A review of adhesion science. Dent Mater. 2010;26(2):e11-6.
3. Mcleod ME, Price RB, Felix CM. Effect of configuration factor on shear bond strengths of self-etch adhesive systems to ground enamel and dentin. Oper Dent. 2010;35(1):84-93.
4. Torres CR, Barcellos DC, Pucci CR, Lima GM, Rodrigues CM, Siviero M. Influence of methods of application of self-etching adhesive systems on adhesive bond strength to enamel. J Adhes Dent. 2009;11(4):279-86.
5. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265-73.
6. Sano H, Takatsu T, Ciucchi B, Homer JA, Matheus WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20(1):18-25.
7. Abdalla AI, El Zohairy AA, Abdel Mohsen MM, Feilzer AJ. Bond efficacy and interface morphology of self etching adhesives to ground enamel. J Adhes Dent. 2010;12(1):19-25.
8. Erickson RL, Barkmeier WW, Latta MA. The role of etching in bonding to enamel: a comparison of self-etching and etch-andrinse adhesive systems. Dent Mater. 2009;25(11):1459-67.
9. Nagayassu MP, Shintome LK, Arana-Chavez VE, Fava M. Microshear bond strength of different adhesives to human dental enamel. J Clin Pediatr Dent. 2011;35(3):301-4.
10. Tay FR, Pashley DH. Water treeing: a potential mechanism for degradation of dentin adhesives. Am J Dent. 2003;16(1):6-12.
11. Ibarra G, Vargas MA, Armstrong SR, Cobb DS. Microtensile bond strength of self-etching adhesives to ground and unground enamel. J Adhes Dent. 2002;4(2):115-24.
12. Patil D, Singbal KP, Kamat S. Comparative evaluation of the enamel bond strength of ‘etch-and-rinse' and ‘all-in-one' bonding agents on cut and uncut enamel surfaces. J Conserv Dent. 2011;14(2):147-50.
13. Sinhoreti MAC, Consani S, Goes MF, Sobrinho LC, Knowles JC. Influence of loading types on the shear strength of the dentinresin interface bonding. J Mater Sci Mater Med. 2001;12(1):39- 44.
14. Davidson CL, Boom G, Arends J. Calcium distribution in human and bovine surface enamel. Caries Res. 1973;7(4):349-59.
15. Edmunds DH, Whittaker DK, Green RM. Suitability of human, bovine, equine, and ovine tooth enamel studies of artificial bacterial carious lesions. Caries Res. 1988;22(6):327-36.
16. Nakamichi I, Iwaku ME, Fusayama T. Bovine teeth as possible substitutes in the adhesion test. J Dent Res. 1983;62(10):1076- 81.
17. Mine A, De Munck J, Vivan Cardoso M, Van Landuyt KL, Poitevin A, Kuboki T, et al. Enamel-smear compromises bonding by mild self-etch adhesives. J Dent Res. 2010;89(12):1505-9.
18. Reis A, Moura K, Pellizzaro A, Dal-Bianco K, Andrade AM, Loguercio AD. Durability of enamel bonding using one-step self-etch systems on ground and unground enamel. Oper Dent. 2009;34(2):181-91.
19. Perdigão J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent Mater. 2010;26(2):e24-37.
20. Osorio R, Monticelli F, Moreira MA, Osorio E, Toledano M. Enamel-resin Bond durability of self-etch and etch & rinse adhesives. Am J Dent. 2009;22(6):371-5.
21. Tsujimoto A, Iwasa M, Shimamura Y, Murayama R, Takamizawa T, Miyazaki M. Enamel bonding of single step self-etch adhesives: influence of surface energy characteristics. J Dent. 2010;38(2):123-30.
22. Hipólito VD, Alonso RC, Carrilho MR, Anauate Netto C, Sinhoreti MA, Goes MF. Microtensile bond strength test and failure analysis to assess bonding characteristics of different adhesion approaches to ground versus unground enamel. Braz Dent J. 2011;22(2):122-8.
23. Lührs AK, Guhr S, Schilke R, Borchers L, Geurtsen W, Günay H. Shear bond strength of self-etch adhesives to enamel with additional phosphoric acid etching. Oper Dent. 2008;33(2):155- 62.
24. Sroczyk-Jaszczyńiska M, Lisiecka-Opalko K, Reszka K, Andersz P. Microscopic evaluation of the tooth-bond-composite interface. Images from SEM: preliminary report. Ann Acad Med Stetin. 2009;55(2):68-70.
25. Perdigão J, Lopes MM, Gomes G. In vitro bonding performance of self-etch adhesives: II--ultramorphological evaluation. Oper Dent. 2008;33(5):534-49.
26. Andrade AP, Shimaoka AM, Russo EMA, Carvalho RCR. Estudo comparativo da resistência de união de sistemas adesivos autocondicionantes com diferentes pHs aplicados ao esmalte e à dentina. RGO - Rev Gaúcha Odontol. 2008;56(2):115-9.
27. Grégoire G, Ahmed Y. Evaluation of the enamel etching capacity of six contemporary self-etching adhesives. J Dent. 2007;35(5):388-97.
28. Pashley DH, Tay FR. Aggressiveness of contemporary self-etching systems: Part II - etching effects on unground enamel. Dent Mater. 2001;17(5):430-44.
29. Sinhoreti MAC, Goes MF, Consani S, Correr Sobrinho L. Avaliação dos sistemas adesivos sobre esmalte: avaliação "in vitro" da resistência ao cisalhamento da união. RGO - Rev Gaúcha Odontol. 2000;48(1):27-30.
30. Skupien JA, Susin AH, Angst PD, Anesi R, Machado P, Bortolotto T, Krejci I. Micromorphological effects and the thickness of the hybrid layer - a comparison of current adhesive systems. J Adhes Dent. 2010;12(6):435-42.
 Endereço para correspondência:
Endereço para correspondência:
GQM MONTEIRO
Universidade de Pernambuco,
Faculdade de Odontologia,
Departamento de Odontologia Restauradora.
Av. General Newton Cavalcanti,
1650, Tabatinga, 54753-220,
Camaragibe, PE, Brasil.
e-mail: gabrielaqueiroz@hotmail.com.br
Recebido: 9/7/2010
Aceito: 16/5/2011













