Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.2 Porto Alegre Abr./Jun. 2012
ORIGINAL / ORIGINAL
Profile of patients with temporomandibular joint disorder: main complaint, signs, symptoms, gender and age
Características dos pacientes com disfunção temporomandibular quanto à queixa principal, sinais, sintomas, sexo e idade
Ivoney Bittencourt CORDEIRO I; Antônio Sérgio GUIMARÃES I
I Faculdade São Leopoldo Mandic, Curso de Odontologia. Rua José Rocha Junqueira, 13, Swift, 13045-755, Campinas, SP, Brasil
ABSTRACT
Objective
This study investigated the most common features of the signs and symptoms of temporomandibular joint disorder and patient profile, including age and gender. The study was done at the First Master Level temporomandibular joint disorder and Orofacial Pain Clinic of São Leopoldo Mandic School of Dentistry, in Campinas, São Paulo, Brazil, to better familiarize general dental surgeons and experts with this disorder.
Methods
This cross-sectional, retrospective study collected data from the records of the first consecutive 200 patients seen at the abovementioned clinic. The collected data included gender, age, main complaints and duration of each complaint. If pain was the main complaint, the affected region and pain intensity were also included.
Results
Most (81%) patients were females with a mean age of 36.5 years, ranging from 9 to 82. Pain was the most common complaint referred by 86% of the sample. The complaint bruxism/clenching had the longest mean duration (82.9 months). Pain was most common in the parotideomasseteric region, referred by 53% of the cases.
Conclusion
Individuals seeking treatment for temporomandibular joint disorder are usually middle-aged females complaining of pain, especially in parotideomasseteric region.
Indexing terms: Epidemiology. Signs and symptoms. Temporomandibular joint disorder.
RESUMO
Objetivo
Avaliar as características dos sinais e sintomas relatados como queixa principal pelos pacientes da clínica do I Curso de Mestrado em Disfunção Temporomandibular e Dor Orofacial da Faculdade São Leopoldo Mandic, correlacionando com sexo, idade e tempo de duração da queixa. Dessa forma, contribuindo com informações que permitam ao cirurgião-dentista, clínico ou especialista, conhecer o perfil destes pacientes.
Métodos
Estudo transversal do tipo retrospectivo onde foram coletados nas fichas dos 200 primeiros pacientes a contar do início das atividades do referido curso os dados referentes ao sexo, idade, queixa principal (podendo haver mais de uma) e duração de cada queixa. Nos casos em que a queixa principal foi dor, foram colhidos na história pregressa da doença atual os dados referentes à região afetada e intensidade da dor.
Resultados
Na amostra estudada 81% dos pacientes que procuraram tratamento foram do sexo feminino; a idade média foi 36,5 anos variando de nove a 82 anos; dor foi a queixa mais frequente, sendo o motivo de procura para tratamento de 86% dos pacientes; a queixa bruxismo/apertamento foi a que apresentou maior tempo médio de duração (82,9 meses); a região dolorosa mais frequentemente citada foi a parotídeo-massetérica, que apareceu em 53% dos pacientes que se queixaram de dor.
Conclusão
Os dados obtidos a partir dessa análise permitem traçar o perfil do paciente que procura atendimento para disfunção temporomandibular como sendo em sua maioria do sexo feminino, adulto jovem, queixando-se de dor, principalmente na região parotídeo-massetérica.
Termos de indexação: Epidemiologia. Sinais e sintomas. Síndrome da disfunção da articulação temporomandibular.
INTRODUCTION
Temporomandibular joint disorder (TMD) consists of a set of signs and symptoms that affect the temporomandibular joint (TMJ), muscles of mastication and the associated structures. The main complaints are pain, limited or changed mandibular movements and joint noise1.
TMD is identified as the main cause of non-dental pain in the orofacial region. The significant pain associated with TMD and the chronic nature of its symptoms have a negative impact on quality of life2-4.
The frequency of TMD signs and symptoms is high in many population groups, especially in women. Variables such as age, main complaint, real need of treatment and association of TMD with contributing factors, such as stress, anxiety, depression and quality of life, continue to be questioned5-9.
Some studies have suggested that the intensity of the signs and symptoms should also be studied to determine how much TMD affects quality of life and the real need of treatment. Epidemiological studies must be interpreted carefully because the presence of signs and symptoms alone does not indicate degree of disability10-11.
Studies of epidemiological nature based on questionnaires compose much of the literature on TMD. In general, epidemiological studies have an important role, since they can be used to guide prevention and control programs. Therefore, epidemiological data are important for estimating TMD rate and distribution12-13.
Knowing the characteristics of patients with TMD, such as gender, most affected age group, and most common signs and symptoms, whether isolated or associated, helps the dental surgeon, clinician or expert to know the profile of these patients, and possibly aid in the diagnoses of TMD-related changes14.
Based on the above, the purpose of this study was to compare the Brazilian reality with the literature and determine the profile of patients with TMD to facilitate their identification and allow clinical use of epidemiological data.
METHODS
The present retrospective, cross-sectional study assessed the records of the first consecutive 200 patients seen at the School São Leopoldo Mandic dental clinic of the master's course on TMD and Orofacial Pain.
The following data were collected from the records: a) gender and age; b) main complaint and respective duration, and reasons for seeking care; c) when pain was the main complaint, the affected region and pain intensity were also collected.
The main complaints of the patient were sometimes recorded as disclosed, so they were interpreted and transcribed as follows: a) noises: snaps, clicks; crepitation (sound of sand or bicycle ratchet); buzz, hum; b) pain; c) limited movements: mouth stuck open (difficulty or inability to close mouth); mouth stuck shut (difficulty or inability to open mouth); irregular movements (tremors, spasms, crooked mouth); d) bruxism/clenching: teeth clenching or grinding while awake or sleeping; e) other changes: partial or complete hearing loss; vertigo/ dizziness; nausea/vomiting; aural fullness; uncomfortable bite, occlusal changes, dental wear; facial asymmetry.
Pain intensity was determined by a visual analogue scale (VAS) with a scale from 1 to 10.
The variables gender, age, complaint duration with the signs and symptoms of TMD, affected regions and pain intensity were statistically assessed and compared. The qualitative variables were represented by absolute (n) and relative (%) frequencies, and the quantitative variables by mean, standard deviation (SD), minimum and maximum. The significance level was set at 5%. All analyses were done by the software SPSS version 12.0 for Windows (Microsoft Corporation). The results were graphed.
This study was approved by the Research Ethics Committee of the São Leopoldo Mandic School, protocol number nº. 06/348.
RESULTS
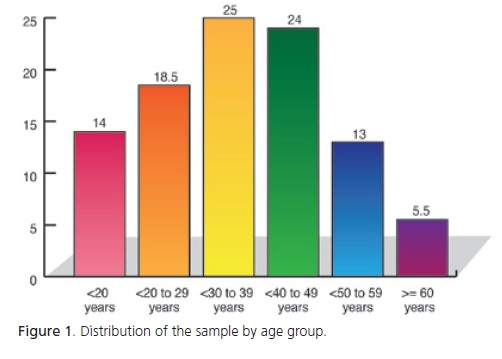
The sample consisted of 200 patients, 162 (81%) females and 38 (19%) males. The mean age was 36.5 years, ranging from 9 to 82 years. Figure 1 shows the distribution of the sample by age group.
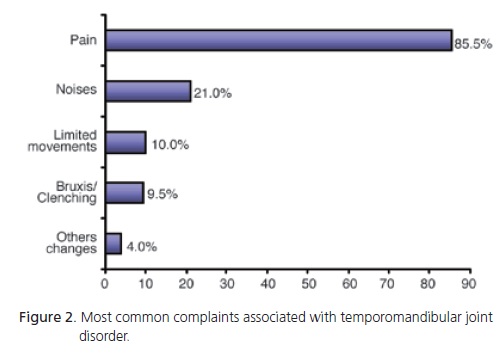
Pain was the most common complaint and the reason that made 86% of the patients seek help. Other complaints were joint noise (21%), limited movements (10%), bruxism/clenching (9%) and other changes (4%). Figure 2 shows the most common complaints.
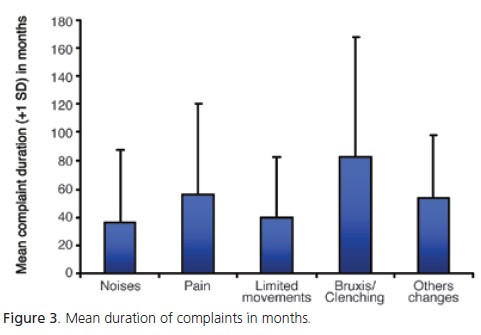
Bruxism/clenching had the longest duration, with a mean duration of 82.9 months (minimum of 4 and maximum of 312 months), followed by pain, other changes, limited movements and noises (Figure 3).
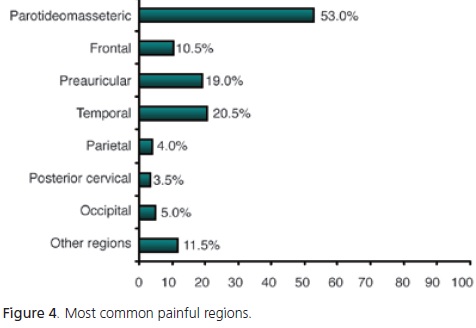
The most common painful areas were the parotideomasseteric region in 53% of the patients, followed by the temporal region (21%), preauricular region (19%) and frontal region (11%) (Figure 4).
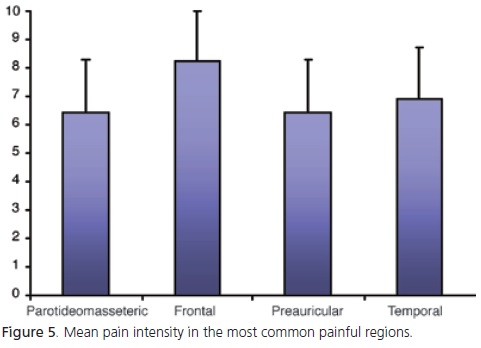
According to VAS, the mean pain intensity for the parotideomasseteric region was 6.4; temporal region, 6.9; auricular region, 6.4; and frontal region, 8.2 (Figure 5).
DISCUSSION
The first epidemiological studies on TMD signs and symptoms were done in Scandinavia and Northern Europe in the early 1970's. Later, many other countries published studies on TMD11.
There are two types of epidemiological studies: casuistic studies include patients who seek care, and random sampling studies include people chosen randomly from the general population15.
The present cross-sectional, retrospective study is a casuistic study of patients who sought treatment for TMD. A total of 200 patients were included, 81% females and 19% males, which is in agreement with Zanettini & Zanettini5, Bonacci et al.16 and Koidis et al.17, who used a similar casuistic methodology and found that females are more likely to seek treatment for TMD.
The prevalence of females seeking treatment for TMD found by the present study has been confirmed by other studies. Population studies with large samples have found that TMD is quite common, with 20% to 88% of all individuals presenting at least one sign or symptom18, and that TMD prevails in females2,19-20.
Bonjardim et al.9 and Wänman & Agerberg21 did not find different prevalences of TMD between male and female adolescents. Bonjardim et al.9 suggested that perhaps some adolescents in their study had not yet undergone all the changes associated with puberty and that the signs and symptoms of TMD are more common in adult women of childbearing age.
The age groups most commonly affected by TMD are 30-39-year-olds (25%) and 40-49-year-olds (24%); together, they compose almost half the patients. Similarly, Helkimo22 reports that TMD prevails in the age group 35- 44 years, and Zanettini & Zanettini5 in the age group 20-40 years. On the other hand, Oliveira et al.3 found TMD to be most common in the age group 20-30 years. This age group ranks third (18.5%) in the present study.
In summary, the youngest and oldest groups have the lowest prevalence of TMD, showing that the signs and symptoms of TMD are uncommon in the first two decades of life, peak between the third and fifth decades and start declining in the sixth decade. These results corroborate those of other studies that report a peak incidence of TMD in young adults8,18,23-24.
Pain was the most common reason for seeking treatment (86%). Greene & Marbach15 and Carlsson18 state in their literature reviews that it is difficult to standardize TMD studies because authors often compare studies with very different experimental designs; for example, studies that rely on questionnaires are often compared with those that rely on clinical examinations. And there are studies that consider joint noise a sign, a symptom, or do not distinguish signs from symptoms. Studies that rely on random sampling find that the most common complaint of individuals with TMD is joint noise9,14,25-26 while studies on individuals with TMD find that the most common complaint is pain5,7,16.
The results of the present study and those listed in the two paragraphs above indicate that joint noise may be in fact the most common sign of TMD in the general population, but not the main reason for seeking treatment. Usually, patients only seek help when they feel pain.
The duration of the sign or symptom that encouraged patients to seek treatment varied from 3 days to 312 months or 26 years (mean=53.64 months or 4.47 years). Felício et al.7 found durations of six months to 23 years (mean=6.26 years). These data suggest that TMD are long-lasting.
Bruxism/clenching presented the longest mean duration (82.9 months), followed by pain (55.7 months), other changes (53.4 months), limited mandibular movements (40 months) and joint noise (36.2 months). Other studies should investigate these cases more thoroughly, for example, retrospectively, to verify if these individuals have ever been submitted to treatment, and prospectively, to assess treatment efficacy or the occurrence of spontaneous remission, as reported by Magnusson et al.24, Onizawa & Yoshida25 and Magnusson et al.27 in their longitudinal studies. These authors also reported that the severity of signs and symptoms varies over time, but rarely become severe.
One limitation of this study is that many patients presented more than one complaint. Although the complaints were grouped separately and 200 patients presented a total of 260 complaints, most of the patients with more than one complaint reported same duration for all of them. Therefore, it is unlikely that these complaints are independent, so the only analysis possible is descriptive analysis based on sign and/or symptom duration. If patients' complaints had different durations, for example, pain for 12 months and buzzing for 24 months, pain was much more common than all other complaints, so statistically it would not be correct to compare groups of very different sizes, for example, pain in 86% of the patients and buzzing in 2.5% of the patients.
TMD usually affects quality of life and a correlation between cause and effect is always being sought. Goddard & Karibe6 studied the prevalence of TMD in an urban and rural population because of the premise that quality of life in rural areas is better, so individuals would be less likely to have TMD. Indeed, they found a higher prevalence of TMD in the urban population and their results were confirmed by Puri et al.28. However, both studies state that studies with more representative samples are necessary to confirm their findings.
Oliveira et al.3 investigated how TMD affects quality of life and found that TMD has a negative impact on work, school, sleep and food intake. The negative impact is mainly caused by pain, which is in fact the most common complaint of patients with TMD who seek treatment.
Pain is described as an unpleasant sensory and emotional experience and is associated with potential or actual tissue damage or described in ways that suggest damage29.
In dentistry, pain may be caused by dental, muscular, joint or neurological conditions within the stomatognathic system, and is considered a symptom2. Pain is most commonly experienced in the parotideomasseteric, temporal, preauricular and frontal regions.
The terminology found in the literature for different head and neck areas varies, so different authors may have used different names for the same region. In other words, the symptom headache may refer to pain in the frontal, temporal, parietal and/or occipital area. TMJ pain, for instance, could be interpreted as pain the preauricular region; likewise, masseter pain could mean pain in the parotideomasseteric region, which is the area overlying the masseter muscle.
Conti2 found that 54% of individuals with TMD also experience headaches, which is similar to the findings of the present study considering that some patients reported pain in the frontal and temporal regions.
The results of this study are also similar to those of Wänman & Agerberg30, who reported that the temporal muscle (temporal region), and TMJ (preauricular region) and temporal muscle (temporal region), respectively, are the most common areas affected by pain.
Most patients of the present study reported pain in the parotideomasseteric region. However, this region has not been mentioned in literature reviews. This may be explained by the different methodologies used by different studies.
Pain intensity was analyzed descriptively as the low representativeness of some regions would compromise the statistical analyses. Hence, only the four most common painful regions were included. The pain intensity reported for most regions was 6 in a scale (VAS) from 1 to 10, which may be considered average. The only exception was the temporal region, which received a score of 8.2. These data are in agreement with those of Oliveira et al.4, who also found average pain intensities despite using a different scale, a weighted scale ranging from 1 to 5.
Pain has a subjective and individual character. However, TMD pain may have a greater impact on quality of life because of its prolonged duration than because of its intensity, since it is hardly disabling.
Finally, since one of the objectives of epidemiological studies is to describe the characteristics of a disease and, repeating the words of Carlsson18, the results of the present study help to elucidate the profile of most patients with TMD: female, aged 20 to 49 years, seeking treatment because of persistent pain in the parotideomasseteric area having lasted roughly 4.7-years, with an intensity of 6.9 in a scale from 1 to 10.
New studies are needed to further investigate the topics covered by the present study, possibly with larger sample sizes and long-term follow-up.
CONCLUSION
Most (81%) individuals who seek treatment for TMD are females with a mean age of 36.5 years. In other words, adults aged 20 to 49 years composed most of the sample.
Pain was the most common complaint, made by 86% of the sample. Complaints had a mean duration of 53.64 months and bruxism/clenching had the longest duration (82.9 months).
Pain was most common in the parotideomasseteric (53%), temporal (20%), preauricular (19%) and frontal (10%) regions. The mean pain intensity on these regions was 6.9 on a scale from 1 to 10 (visual analogue scale).
Collaborators
IB CORDEIRO conceived the study and helped to write the manuscript. AS GUIMARÃES supervised the study and helped to write the manuscript.
REFERENCES
1. Rollman GB, Gillespie JM. The role of psychosocial factors in temporomandibular disorders. Curr Rev Pain. 2000;4(1):71-81. [ Links ]
2. Conti PCR. Avaliação da prevalência das dores de cabeça primárias e seu relacionamento com sintomas de desordens temporomandibulares do campus da USP, na cidade de Bauru - SP. Rev Dent Press Ortodon Ortopedi Facial. 2003;8(2):49-56.
3. Oliveira AS, Bermudez CC, Souza RA, Souza CMF, Dias EM, Castro SCE et al. Impacto da dor na vida de portadores de disfunção temporomandibular. J Appl Oral Sci. 2003;11(2):138-43.
4. Oliveira AS, Bermudez CC, Souza RA, Souza CMF, Castro CES, Berzin F. Avaliação multidimensional da dor em portadores de desordem temporomandibular utilizando uma versão brasileira do questionário McGill de dor. Rev Bras Fisioter. 2003;7(2):151-8.
5. Zanettini I, Zanettini UM. Desordens temporomandibulares: estudo retrospectivo de 150 pacientes. Rev Cient AMECS. 1999;8(1):9-15.
6. Goddard G, Karibe H. TMD prevalence in rural and urban native american populations. Cranio. 2002;20(2):125-8.
7. Felício CM, Mazzetto MO, Bataglion C, Silva MAMR, Hotta TH. Desordem temporomandibular: análise da freqüência e severidade dos sinais e sintomas antes e após a placa de oclusão. J Bras Ortodon Ortop Facial. 2003;8(43):48-57.
8. Pereira Junior FJ, Vieira AR, Prado R, Miasato JM. Visão geral das desordens temporomandibulares. RGO - Rev Gaúcha Odontol. 2004;52(2):117-21.
9. Bonjardim LR, Gavião MB, Pereira LJ, Castelo PM, Garcia RC. Signs and symptoms of temporomandibular disorders in adolescents. Braz Oral Res. 2005;19(2):93-8. doi: 10.1590/ S1806-83242005000200004.
10. Mathew TG, Mathew T. Taking care of the challenging tension headache patient. Curr Pain Headache Rep. 2011;15(6):444-50. doi: 10.1007/s11916-011-0223-1.
11. Carlsson GE, Magnusson T, Guimarães AS. Tratamento das disfunções temporomandibulares na clínica odontológica. São Paulo: Quintessence; 2006.
12. Allori AC, Chang CC, Fariña R, Grayson BH, Warren SM, McCarthy JG. Current concepts in pediatric temporomandibular joint disorders: Part 1. Etiology, epidemiology, and classification. Plast Reconstr Surg. 2010;126(4):1263-75.
13. Fonseca DM, Bonfante G, Valle AC, Freitas STF. Diagnóstico pela anamnese da disfunção craniomandibular. RGO - Rev Gaúcha Odontol. 1994;42(1):23-8.
14. Serman RJ, Conti PCR, Conti JV, Salvador MCG. Prevalência de disfunção temporomandibular em pacientes portadores de prótese total dupla. J Bras Oclusão ATM dor Orofac. 2003;3(10):141-4.
15. Greene CS, Marbach JJ. Epidemiologic studies of mandibular dysfunction: a critical review. J Prosthet Dent. 1982;48(2):184- 90.
16. Bonacci CE, Syrop SB, Gold N, Israel H. Temporomandibular/ facial pain. An epidemiological report. N Y State Dent J. 1992;58(5):30-3.
17. Koidis PT, Zarifi A, Grigoriadou E, Garefis P. Effect of age and sex on craniomandibular disorders. J Prosthet Dent. 1993;69(1):93- 101. doi: 10.1016/0022-3913(93)90247-L.
18. Carlsson GE. Epidemiological studies of signs and symptoms of temporomandibular joint-pain-dysfunction: a literature review. Aust Prosthodont Soc Bull. 1984;14:7-12.
19. Agerberg G, Carlsson GE. Functional disorders of the masticatory system. I. Distribution of symptoms according to age and sex as judged from investigation by questionnaire. Acta Odontol Scand. 1972;30(6):597-613.
20. Solberg WK, Woo MW, Houston JB. Prevalence of mandibular dysfunction in young adults. J Am Dent Assoc. 1979;98(1):25- 34.
21. Wanman A, Agerberg G. Mandibular dysfunction in adolescents. I. Prevalence of symptoms. Acta Odontol Scand. 1986;44(1):47- 54.
22. Helkimo M. Epidemiological surveys of dysfunction of the masticatory system. Oral Sci Rev. 1976;7:54-69.
23. LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8(3):291-305. doi: 10.1177/10454411970080030401.
24. Magnusson T, Egermark I, Carlsson GE. A prospective investigation over two decades on signs and symptoms of temporomandibular disorders and associated variables: a final summary. Acta Odontol Scand. 2005;63(2):99-109.
25. Onizawa K, Yoshida H. Longitudinal changes of symptoms of temporomandibular disorders in japanese young adults. J Orofacial Pain. 1996;10(2):151-6.
26. Rauhala K, Oikarinen KS, Järvelin MR, Raustia AM. Facial pain and temporomandibular disorders: an epidemiological study of the Northern Finland 1966 Birth Cohort. Cranio. 2000;18(1):40- 6.
27. Magnusson T, Egermark I, Carlsson GE. A longitudinal epidemiologic study of signs and symptoms of temporomandibular disorders from 15 to 35 years of age. J Orofac Pain. 2000;14(4):310-9.
28. Puri P, Diamond M, Solomowitz BH, Sher MR. Temporomandibular disorders in an urban population. An epidemiological study. N Y State Dent J. 1994;60(7):42-3.
29. Merksey H, Bogduk N. Classification of chronic pain: descriptions of chronic Pain syndromes and definitions of pain terms. 2nd ed. Seattle: IASP Press; 1994.
30. Wanman A, Agerberg G. Mandibular dysfunction in adolescents. II. Prevalence of signs. Acta Odontol Scand. 1986;44(1):55-62.
 Correspondence to:
Correspondence to:
IB CORDEIRO
e-mail: ivoney@uol.com.br
Received on: 10/2/2011
Final version resubmitted on: 6/8/2011
Approved on: 28/1/2012













