Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.3 Porto Alegre Jul./Set. 2012
ORIGINAL / ORIGINAL
Use of 0.25% and 0.025% peracetic acid as disinfectant agent for chemically activated acrylic resin: an in vitro study
Uso do ácido peracético a 0,25% e a 0,025% como agente desinfetante de resina acrílica ativada quimicamente: estudo in vitro
Luciana REIS I; Artêmio Luiz ZANETTI I; Osmar Vieira CASTRO JUNIOR I; Elizabeth Ferreira MARTINEZ II
I Faculdade São Leopoldo Mandic, Curso de Odontologia, Programa de Pós-Graduação em Prótese. Campinas, SP, Brasil
II Instituto e Centro de Pesquisas São Leopoldo Mandic, Programa de Pós-Graduação em Patologia. Rua José Rocha Junqueira, 13, Swift, 13045-755, Campinas, SP, Brasil
ABSTRACT
Objective
This in vitro study evaluated the disinfection action of peracetic acid on chemically activated acrylic resin.
Methods
Sixty chemically activated acrylic resin specimens were contaminated with Candida albicans (30) and Bacillus subtilis (30) for 15 minutes. Next, specimens were divided into Control Group (Group 0) and Test Group (T) for each studied microorganism. The antimicrobial effect of Proxitane® Alfa (Thech Desinfecção, São Paulo, Brazil), containing 0.25% and 0.025% concentrations of peracetic acid was verified after 1, 3, 5 and 10 minutes of exposure. The specimens were transferred to saline solution for 5 minutes, homogenized and aliquots of 100μL were plated on BHI and Sabouraud Dextrose agar. After incubation at 37ºC/24h, the number of CFU/mL recovered from each specimen was obtained.
Results
The 0.025 % peracetic acid was effective against B. subtilis only after 10 minutes and against C. albicans after 3 minutes of exposure. At 0.25% concentration, the solution showed fungicidal and bactericidal efficacy after 1 minute of exposure.
Conclusion
The 0.25% peracetic acid was shown to be efficient for disinfection of chemically activated acrylic resins.
Indexing terms: Acrylic resin. Complete denture. Disinfection. Peracetic acid.
RESUMO
Objetivo
Avaliar in vitro a ação desinfetante do ácido peracético sobre resina acrílica quimicamente ativada.
Métodos
Sessenta corpos de prova em resina acrílica quimicamente ativada foram contaminados em suspensão de Candida albicans (n=30) e Bacillus subtilis (n=30) por 15 minutos. A seguir, os corpos de prova foram divididos em grupo Controle (Grupo 0), com 6 espécimes e Grupo teste composto por 24 espécimes para cada microrganismo estudado. Proxitane® Alfa (Thech Desinfecção, São Paulo, Brasil) ácido peracético foi testado nas concentrações de 0,25% e 0.025%, após 1, 3, 5 e 10 minutos de exposição. Após, cada corpo de prova foi transferido para solução fisiológica por 5 minutos, homogeneizados e alíquotas de 100 μL foram semeadas em duplicata, em BHI e Sabouraud Dextrose ágar. Após incubação a 37ºC / 24 horas, determinou-se o número de UFC/ml recuperado de cada espécime.
Resultados
Na concentração de 0,025%, o ácido peracético mostrou efeito frente a Bacillus subtilis apenas após 10 minutos e para Candida albicans, após 3 minutos. Na concentração de 0,25%, a solução mostrou efeito fungicida e bactericida após apenas 1 minuto de exposição.
Conclusão
O ácido peracético a 0,25% demonstrou-se eficaz na desinfecção de resina acrílica quimicamente ativada.
Termos de indexação: Resinas acrílicas. Prótese total. Desinfecção. Ácido peracético.
INTRODUCTION
The acrylic resins widely used in dental prosthetic devices are heat sensitive materials that cannot be sterilized by autoclaving or in ovens, and must be disinfected, or preferably sterilized by chemical or mechanical agents. According to Anusavice1, acrylic resin has liquid sorption capacity and when it comes into contact with the oral cavity, it is capable of absorbing and adsorbing saliva and blood, thereby becoming a vehicle of cross contamination.
The disinfection of prosthetic devices is an important stage in preventing cross contamination among patients, dentists and laboratory technicians2-3 because a series of not sterilized materials and instruments are used in denture manufacture4-6.
Denture immersion in chemical products has been shown to be more efficient than mechanical brushing7-9. Thus, many studies have recommended efficient chemical solutions, such as glutaraldehyde, sodium hypochlorite and chlorhexidine9-12. However, it is highlighted in the literature that the use of these solutions is not recommended for acrylic resins disinfection. Glutaraldehyde is not safe due to its toxicity3, and sodium hypochlorite is hardly effective as antimicrobial agent, and at residual concentration13, may affect the surface roughness of acrylic resin causing degradation of the product14. In contrast, chlorhexidine is considered an effective disinfectant agent, however it may cause staining14 and dimensional alteration of acrylic resin15.
The use of peracetic acid is being investigated as a feasible alternative of disinfectant solution. This product is of promoting to promote an effective disinfectant and sterilizing action without affecting the physical-chemical properties of acrylic resin18 or compromising the individual's health. For McDonell & Russell16, peracetic acid is considered a more powerful biocide than hydrogen peroxide, as it is sporicidal17, bactericidal, virucidal and fungicidal properties at low concentrations (<0.3%). Peracetic acid probably denatures proteins and enzymes and increases the cell wall permeability by rupturing the hydrated sulphate (-SH) and sulphur (S-S) bond. Thus, peracetic acid is effective against a wide variety of microorganisms18, oxidizing vital components for the survival of viruses, bacteria, fungi and spores.
It is necessary to provide a clean and cross contamination-free9-10 denture to the patient which does not compromise the individual's health. Due to the importance of this subject, this research was developed to verify in vitro the efficacy of the use of peracetic acid as a disinfectant agent for chemically activated acrylic resin, in different time intervals and concentrations.
METHODS
To conduct this research, 60 test specimens were manufactured of chemically activated acrylic resin powder and liquid (Jet® Artigos Odontológicos Clássico Ltda., São Paulo, Brazil), which are replicas of an old mandibular complete denture19 made with the use of a laboratory silicone matrix (Labormass - Ruthbras®, São Paulo, Brazil) and pressed in a muffle (Vipi-STG®, São Paulo, Brazil). The purpose of the technical procedure performed for each test specimen was to simulate the conditions normally used for polishing a mandibular complete denture. All the parts were polished with fresh pumice stone and water solution. The tests specimens were not decontaminated with any disinfectant solution since the purpose was to evaluate the efficacy of peracetic acid in microbial reduction. For this aim, the test specimens were contaminated with a suspension of Candida albicans and Bacillus subtilis at the concentration of 15X108 microorganisms/ml (Factor No. 5 Mc Farland's Nephelometric Scale, Nefelobac®, São Paulo, Brazil) in 100 ml of sterilized physiological solution. The investigation was conducted to observe the behavior of the disinfectant against a fungus commonly found as contaminant on acrylic resin, as well as a spore-producing microorganism, due to the high resistance of spores to chemical agents.
Test specimen manufacture
Four measures of extra hard laboratory silicone (Labormass - Ruthbras®, São Paulo, Brazil) was mixed with catalyzer to model the internal part of the denture, covering around 2 mm of the outer edge. After the silicone was cured, the edges were trimmed with a stiletto and "V" shaped depressions were made in the lateral platform. After this, the silicone that would come into contact with the second part to be molded was smeared with vaseline. Afterwards, the external part of the denture was molded, so that the silicone would penetrate into the "V" shaped depressions.
The first molded part (which corresponds to the internal part of the denture) was included in the base of the muffle with 120g of common plaster (Asfer®, São Paulo, Brazil). After crystallization, the plaster was isolated with a separating liquid. Next, the second molded part (which corresponds to the external part of the denture) was fitted into the first part that had already been included. The base was fitted into the counter-muffle and the 4 screws were fastened. The plaster was prepared using 180g of common plaster (Asfer®, São Paulo, Brazil) and poured in through the counter-muffle orifice. In the beginning, circular movements were made with the muffle so that the plaster would penetrate into all the crevices. Then, with the muffle supported on the bench, it was completely filled with plaster. After plaster crystallization, the 4 screws were removed, the base separated from the counter-muffle, and the plaster was isolated with separating liquid.
Chemically activated acrylic resin (Jet® Artigos Odontológicos Clássico Ltda., São Paulo, Brazil) was mixed in accordance with the manufacturer's instructions. For each test specimen, 18 parts of powder (14g) to 7.2 (plastic cup for measuring monomer) of liquid (10 ml) of acrylic resin were measured. The parts of the muffle that would come into contact with the resin were previously smeared with vaseline. After preparing the mixture, when the resin reached the sticky stage, it was pressed into the muffle. Then the muffle was closed, the screws were fastened, and when the resin reached the plastic stage, it was pressed at 1000 kgF/F (Vipi Delta hydraulic press, Delta®, São Paulo, Brazil). After completed the polymerization time (30 minutes), the 4 screws were removed, the muffle was opened and the clone removed. The excesses were removed and the test specimen was polished and finished. The same procedure was used to make the other test specimens. After manufacture, the test specimens were polished (Polidor VH® Equipamentos, Araraquara, Brazil).
Microbiological analysis
For this study, strains of Candida albicans ATCC 10231 (Ca) and Bacillus subtilis ATCC 19659 (Bs), both obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) were used.
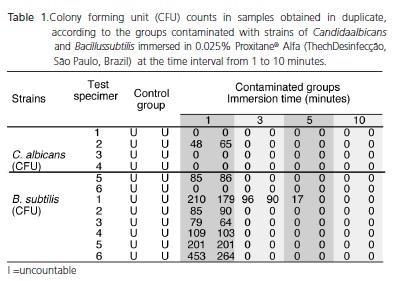
Of the total of 60 test specimens manufactured, 30 were immersed in 100 ml of Candida albicans (Ca) suspension and 30 in Bacillus subtilis (Bs) suspension, at the concentration of 15X108 cells/ml for 5 minutes. Six test specimens contaminated with each microorganism were used as positive control, and were transferred to physiological solution. The remaining 48 specimens were immersed in 0.25% and 0.025% Proxitane® Alfa Sterilization solutions (Thech Desinfecção®, São Paulo, Brazil) for time intervals of 1, 3, 5 and 10 minutes. After the immersion time, each test specimen was submerged in sterile saline solution for 5 minutes and homogenized. Aliquots of 100 μL of controls and tests were plated on agar BHI (Brain Heart Infusion) for B. subitilis and on agar Sabouraud for C. albicans and incubated at 37°C for 24 hours. The colony forming units (CFU/ml) were determined and the percentage of microorganism inhibition by peracetic acid was determined. The experiments were carried out in a blind fashion in duplicate.
Statistical analysis was performed using ANOVA followed by the Tukey test using the SAS 8.2 statistical program (SAS Institute, Cary, NC, USA). The level of significance was 5%.
RESULTS
The results demonstrated that on 0.025% peracetic acid concentration, growth of C. albicans until 1 minute as well as B. subtilis until 5 minutes of disinfectant exposure was observed. After these periods, no microbial growth was observed (Table 1). In contrast, no growth was observed in the specimens contaminated with strains of C. albicans and B. subtilis by immersion in 0.25% peracetic acid in all the studied time intervals.
ANOVA statistical analysis followed by the Tukey test showed no statistically significant difference between the studied groups (p>0.05%).
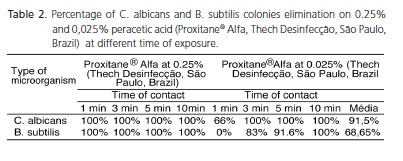
Table 2 summarizes the average percentage of the obtained results. 100% of both studied microorganisms were eliminated when exposed in 0,25% peracetic acid. On the other hand, at 0,025% peracetic acid, 91.5% of the C. albicans strains and 68,65% of B. subtilis strains were eliminated.
DISCUSSION
The development of disinfectant chemical solutions that are capable of maintaining dentures free of plaque with a daily immersion of 15 or 30 minutes, and that do not affect the color or surface of acrylic resin dentures is recommended. In this respect, Thamlikitkul et al.18 showed that the use of 0.2% peracetic acid for disinfection did not significantly alter the sorption, solubility and microhardness properties of heat polymerized and chemically activated acrylic resins. Based on this, the present in vitro study aimed to evaluate the microbial effect of peracetic acid in two concentrations, after different time intervals.
The present results demonstrated the efficacy of disinfection, as well as, the fungicidal and sporicidal effect of 0.25% peracetic acid after 1 minute of exposure to the product. These findings are also in accordance with Baldry20, which showed the rapid activity of peracetic acid against sporulated bacteria and yeasts after only 1 minute. In one of the first studies published in the literature about peracetic acid, Greenspan & MacKeller21 indicated the high bactericidal and fungicidal activity after application of peracetic acid for the effective washing of fruit and vegetables. In contrast, some studies have reported that the fungicidal and sporicidal effects of peracetic acid at the concentration of 0.2% demands at least 5 minutes of exposure to the product21,25-26.
In respect to the effect of diluted peracetic acid, Penna24 affirmed that peracetic acid is bactericidal, fungicidal, virucidal, micobactericidal and sporicidal even at low concentrations (0.001% to 0.2%). The 0.025% concentration of peracetic acid used in the present study is included in this range of sporicidal effect at low concentration tested and proved. Leaper17 obtained a sporicidal effect at 0.04% at 40°C, and Sagripanti & Bonifacino25 at 0.03%, at 20°C as for 30 minutes. Nevertheless, our in vitro results are in disagreement with the records of Greenspan & MacKeller21 who demonstrated that the sporicidal effect of peracetic acid was obtained only at the concentration of 0.3%.
In the tests with Proxitane® Alfa (Thech Desinfecção, São Paulo, Brazil) diluted to 0.025%, with regard to time of exposure, it was also possible to observe that peracetic acid demonstrated a fungicidal effect as from 3 minutes and sporicidal effect as from 10 minutes. Its sporicidal effect was equal to the time of exposure in the tests of Svidzinski et al.26 who also proved that paracetic acid had a sporicidal effect as from the time of 10 minutes, although it had been tested at another concentration: diluted to 0.1%. However, it is not possible to compare with the fungicidal and sporicidal effects obtained by Greenspan & MacKellar21 since they observed these effects after 24 hours of exposure to the product. This also occurred with Sagripanti & Bonifacino25 who observed the sporicidal effect of 0.03% peracetic acid only as from a time of contact of 30 minutes.
As regards the toxicity of paracetic acid, the products of its decomposition are considered safe and harmless (acetic acid and oxygen) that decomposed into non toxic products (oxygen and water)21. Peracetic acid remains active even in the presence of peroxidases and organic matter24; it is non allergenic and it is considered a slight irritant22. Nevertheless, diluted acids such as acetic acid are corrosive14. Due to this disadvantage, and to the excellent fungicidal and sporicidal performance in this study of 0.25% peracetic acid, the following dilution of 0,025% of the product was tested in order to reduce its toxicity. The long-term action of 0.2% peracetic acid was evaluated by Muller et al.22 on the dorsal skin, oral and vaginal mucosa of rabbits after 1 year. The histological exams showed no inflammation, no scar formation, and no risk of dysplasias that could detect the carcinogenic action of peracetic acid. Nevertheless, the authors suggested that the permanent use of peracetic acid in the disinfection of hands might possibly lead to the risk of depilation, and it needs to be tested dermatologically. Therefore, dermatological studies are also necessary to assess and compare the risk of toxicity of 0.25% and 0.025% peracetic acid.
In summary, the immersion of chemically activated acrylic resin dentures in 0.25% peracetic acid for 1 minute and in 0.025% for 10 minutes is recommended as well as effective to promote decontamination in order to deliver them in a clean condition to the patient.
CONCLUSION
The in vitro obtained results demonstrated the efficacy of disinfection of chemically activated acrylic resin by immersion in peracetic acid, mainly on 0.25% concentration.
Collaborators
L REIS was responsible for setting up the research project, literature study, performing the methodology and writing the article. AL ZANETTI guided the research and participated in writing the article. OV CASTRO JUNIOR was the originator of the research, responsible for the first part of the methodology in the field of Prosthesis, supervising the practice developed, interpreting the results and preparing the discussion and conclusion, and writing the article. EF MARTINEZ was responsible for the second part of the methodology in the field of Microbiology, supervising the practice developed, interpreting the results and preparing the discussion and conclusion, and writing the article.
REFERENCES
1. Anusavice KJ. Phillips Materiais dentários. 10ª ed. Rio de Janeiro: Guanabara Koogan; 1998. [ Links ]
2. Katberg Jr JW. Cross-contamination via the prostodontic laboratory. J Prosth Dent. 1974;32(4):412-9.
3. Maranhão KM, Lopes TC, Esteves RA. Biossegurança em prótese dentária: proposta de protocolo. Parte III. PCL. 2006;8(42):363-9.
4. Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosth Dent. 1990;64(2):235-7. doi: 10.1016/0022-3913(90)90185-F.
5. Verran J, Kossar S, McCord JF. Microbiological study of selected risk areas in dental technology laboratories. J Dent. 1996;24(1- 2):77-80. doi: 10.1016/0300-5712(95)00052-6.
6. Cotrim LEF, Santos EM, Jorge AOC. Procedimentos de biossegurança realizados por cirurgiões-dentistas e laboratórios durante a confecção de próteses dentárias. Rev Odontol UNESP. 2001;30(2):233-44.
7. Chan ECS, Iugovaz I, Siboo R, Bilyk M, Barolet R, Amsel R, et al. Comparison of two popular methods for removal and killing of bacteria from dentures. J Can Dent Assoc. 1991;57(12):937-9.
8. Assery M, Sugrue PC, Graser GN, Eisenberg AD. Control of microbial contamination with commercially available cleaning solutions. J Prosth Dent. 1992;67(2):275-7. doi: 10.1016/0022- 3913(92)90467-O.
9. Rudd RW, Senia ES, McCleskey FK, Adams ED. Sterilization of complete dentures with sodium hypochlorite. J Prosth Dent. 1984;51(3):318-21. doi: 10.1016/0022-3913(84)90212-9.
10. Shen C, Javid NS, Colaizzi FA. The effect of glutaraldehyde base disinfectants on denture base resins. J Prosth Dent. 1989;61(5):583-9. doi: 10.1016/0022-3913(89)90281-3.
11. Bell JA, Brockmann SL, Feil P, Sackuvich. The effectiveness of two disinfectants on denture base acrylic resin with an organic load. J Prosth Dent. 1989;61(5):580-3. doi: 10.1016/0022- 3913(89)90280-1.
12. Chau VB, Saunders TR, Pimsler M, Elfring DR. In depth disinfection of acrylic resins. J Prosth Dent. 1995;74(3):309-13. doi: 10.1016/S0022-3913(05)80140-4.
13. Souza JB, Daniel, LA. Comparação entre hipoclorito de sódio e ácido peracético na inativação de E. coli, colifagos e C. perfringens em água com elevada concentração de matéria orgânica. Eng Sanit Ambient. 2005;10(2):111-7. doi: 10.1590/ S1413-41522005000200004.
14. Budtz-Jorgensen E. Material and methods for cleasing dentures. J Prosth Dent. 1979;42(6):619-23. doi: 10.1016/0022- 3913(79)90190-2.
15. Hiraishi N, Yiu CK, King NM, Tay FR, Pashley DH. Chlorhexidine release and water sorption characteristics of chlorhexidineincorporated hydrophobic/hydrophilic resins. Dent Mater. 2008;24(10):1391-9. doi: 10.1016/j.dental.2008.03.011.
16. McDonnel G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147-79.
17. Leaper S. Influence of temperature on the synergistic sporicidal effect of peracetic acid plus hydrogen peroxide on Bacillus subtilis. Food Microbiol. 1984;1(3):199-203. doi: 10.1016/0740- 0020(84)90034-0.
18. Thamlikitkul V, Trakulsomboon S, Louisirirotchanakul S, Chaiprasert A, Foongladda S, Thipsuvan K, et al. Microbial killing activity of peracetic acid. J Med Assoc Thai. 2001;84(10):1375- 82.
19. Gomes T, Gomes FL, Castro Jr OV. Técnica da clonagem em prótese total. PCL. 2003;5(24):101-8.
20. Baldry MGC. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J App Bacteriol. 1983;54(3):417-23.
21. Greenspan FP, MacKeller DG. The application of peracetic acid germicidal washes to mold control of tomatoes. Food Technol. 1951;6:95-7.
22. Muller P, Raabe G, Hörold J, Juretzek U. Action of chronic peracetic acid (Wofasteril®) administration on the rabbit oral mucosa, vaginal mucosa and skin. Exp Pathol. 1988;34(4):223-8.
23. Chassot ALC, Poisl MI, Samuel SMW. In vivo and in vitro evaluation of the efficacy of a peracetic acid-based disinfectant for decontamination of acrylic resins. Braz Dent J. 2006;17(2):117- 21. doi: 10.1590/S0103-64402006000200006.
24. Penna TCV. Desinfecção e esterilização química. Métodos de desinfecção e esterilização. 2ª ed. São Paulo: Atheneu; 2005. p. 133-165 [citado 2011 Jun 10]. Disponível em: <http://www. fcf.usp.br/Departamentos/FBT/HP_Professores/Penna/Livro/Desinfeccao_e_Esterilizacao_Quimica_Capitulo08.pdf>.
25. Sagripanti JL, Bonifacino A. Comparative sporicidal effects of liquid chemical agents. Appl Env Microbiol. 1996;62(2):545-51.
26. Svidzinski AE, Posseto I, Pádua RAF, Tavares TR, Svidzinski TIE. Eficiência do ácido peracético no controle de Staphylococcus aureus meticilina resistente. Cienc Cuid Saúde. 2007;6(3):312-8.
 Correspondence to:
Correspondence to:
EF MARTINEZ
e-mail: efmartinez@ig.com.br
Received on: 14/12/2010
Final version resubmitted on: 8/9/2011
Approved on: 3/1/2012













