Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.3 Porto Alegre Jul./Set. 2012
ORIGINAL / ORIGINAL
Mouthrinses: active ingredients, pharmacological properties and indications
Enxaguatórios bucais: princípios ativos, propriedades farmacológicas e indicações
Danilo Barral de ARAUJO I; Elisângela de Jesus CAMPOS I; Isis Henriques de Almeida BASTOS II; Daniel Miranda de PAULA II; Edval Reginaldo TENÓRIO JUNIOR II; Roberto Paulo Correia de ARAUJO I
I Universidade Federal da Bahia, Instituto de Ciências da Saúde. Av. Reitor Miguel Calmon, s/n., Canela, 40110-100, Salvador, BA, Brasil
II Universidade Federal da Bahia, Faculdade de Odontologia. Salvador, BA, Brasil
ABSTRACT
Objective
To evaluate the formulations of 29 mouthrinses marketed in the city of Salvador, Bahia, Brazil, in 2011, with respect to active principles - antimicrobial-agents - and other components.
Methods
Data collection was performed in commercial wide circulation. Product labels were evaluated, information recorded, and afterwards data were complemented by consulting the scientific literature.
Results
Of the three chlorhexidine-containing mouthrinses, two contained 0.12% chlorhexidine gluconate or digluconate formulated in a free base concentration of chlorhexidine 0.067%, while the third did not report the concentration. Only two mouthwashes contained the antimicrobial agent triclosan at 0.03% concentration associated with PVA Gantrez copolymer at 0.2% concentration to stabilize it. Cetylpyridinium chloride is an antiseptic substance present in most mouthwashes available on the market, while products containing essential oils as active principles in their formulations, usually associate thymol, menthol, eucalyptol and methyl salicylate. Irrespective of the antibacterial agent, the mouthrinses typically have similar complementary substances, especially the addition of fluoride ions.
Conclusion
There are a a wide variety of mouthrinses containing antimicrobial agents in a variety of different spectra on the Market, but no data are provided on other substances added to them. Among antimicrobial agents, the most frequently found in mouthwashes was cetylpyridinium chloride. It is important for the dentist to have adequate knowledge of the spectrum of action of each antimicrobial agent, in order to prescribe the most appropriate type in each case.
Indexing terms: Bacteria. Cetylpyridinium. Chlorhexidine. Oral health. Triclosan.
RESUMO
Objetivo
Avaliar a constituição de 29 enxaguatórios, comercializados na cidade do Salvador, Bahia, Brasil, em 2011, relacionado aos princípios ativos-agentes antimicrobianos - além dos demais componentes.
Métodos
A coleta das informações foi realizada em estabelecimentos comerciais de ampla circulação. Para tanto foram avaliados os rótulos dos produtos, registradas as informações, seguindo-se de complementação dos dados mediante consultas à literatura científica.
Resultados
Dos três enxaguatórios contendo clorexidina, dois contêm gluconato de clorexidina a 0,12% ou digluconato formulado para uma base livre de clorexidina na concentração de 0,067%, enquanto que o terceiro não informa a concentração. Apenas dois colutórios possuem o agente antimicrobiano triclosan na concentração de 0,03% associado ao copolímero PVA Gantrez na concentração de 0,2% para estabilizá-lo. O cloreto de cetilpiridínio é a substância anti-séptica que está presente na maioria dos enxaguatórios veiculados no mercado, enquanto que os produtos que contêm em suas formulações os óleos essenciais como princípios ativos, geralmente associam timol, mentol, eucaliptol e salicilato de metila. Independente do agente antibacteriano, os enxaguatórios em geral possuem substâncias complementares semelhantes, com destaque à adição o íon fluoreto.
Conclusão
O mercado apresenta uma ampla variedade de enxaguatórios contendo relativa variedade de agentes antimicrobianos de diferentes espectros, contudo são limitadas as informações sobre as demais substâncias adicionadas aos mesmos; dentre os agentes antimicrobianos aquele encontrado com maior freqüência nos enxaguatórios é o cloreto de cetilpiridínio; é importante que o cirurgião-dentista tenha conhecimento adequado do espectro de ação de cada agente antimicrobiano a fim de prescrever o mais apropriado a cada caso.
Termos de indexação: Bactérias. Cetilpiridínio. Clorexidina. Saúde bucal. Triclosan.
INTRODUCTION
Mouthwashes have been used in the chemical control of bacterial plaque, a structure characterized as a dense, uncalcified bacterial mass, which serves as a bacterial deposit and that is adhered to the tooth, acquired pellicle, dental calculus and to other structures in the oral cavity1-2. These vehicles are used as facilitators to spread active medication compounds for treating specific diseases such as caries and periodontal disease and they are an adjuvant to the mechanical procedures of brushing3-4.
Mouthwashes consist of a mixture containing the active component, often antimicrobial component, water and(or) ethanol, surfactants, humectants and flavorings5. The most frequently used pharmacological antimicrobial agents are: chlorhexidine, cetylpyridinium chloride and triclosan in addition to essential oils2-3,6. Most antimicrobial agents on the market promote the disruption of the cell wall, inhibit the enzyme complexes, cumilnating in the impairment of bacterial metabolic activities3.
Although such active principles have bactericidal and bacteriostatic action of greater or lesser intensity, they have led significant segments of society to believe that their use is sufficient to ensure satisfactory oral hygiene. It is extremely important for dental professionals to draw their patients' attention to the need for performing proper oral hygiene, and the fact that mouthwashes are only adjuvants1.
An ideal antimicrobial agent should have antiplaque potential and must necessarily cause reduction of bacterial adhesion to tooth surfaces and oral mucosa; inhibit growth and proliferation of microorganisms; inhibit the formation of intercellular plaque matrix, modify bacterial metabolism to reduce the formation of cytotoxic products; and modify the ecology of biofilm to favor the development of a less pathogenic oral microflora6.
Among the above-mentioned compounds, chlorhexidine is the most commonly used active antiseptic to control plaque2-4,7-8. It is regarded as the gold standard in comparison with the other chemical agents that have similar action due to the pharmacological effect of inhibiting dental biofilm.9 One of the major advantages of using it its broad antimicrobial spectrum, acting on gram-positive and gram-negative bacteria, as well as the prolonged and continuous substantivity even in the presence of blood and other body fluids10. It is a chemotherapeutic agent consisting of two chlorinated phenolic rings and two bis-biguanide groups symmetrically connected by a hexamethylene chain2,3,8. Being considered a stable agent as once ingested it is excreted by the normal pathways, only a small percentage is retained in the body, which does not cause toxicity8,11. The mechanism of action starts from the link between this antiseptic agent and the bacterial cell wall due to the adsorption of the positive charges of this molecule to the negative charges of the bacterial surfaces. This phenomenon makes surface more permeable allowing this agent to penetrate into the cytoplasmic medium and consequently cause disruption of the cell membrane with leakage of cellular structures of the microorganism2,6. Despite the effectiveness of chlorhexidine, it has several side effects, among which pigmentation of teeth and tongue and taste and sensory changes are outstanding2,7.
Another frequently used antimicrobial agent in the form of mouthwash is cetylpyridinium chloride, also called hexadecylpyridinium chloride12. This agent is chemically classified as a quaternary, monocationic2,5-6,9 and surfactant6 ammonium compound that acts primarily on Gram-positive bacteria and yeasts3,6. With regard to the spectrum of action of cetylpyridinium chloride, the scientific literature is discordant as some authors claim that this drug acts only on gram-positive bacteria6, while others claim that it affects both gram-positive and gram-negative as well as yeasts12. However, there is consensus among authors regarding its mechanism of action as they admit that the effect of this antiseptic is related to the increase in permeability of the bacterial cell wall, favoring its lysis, reduction in metabolism and interruption of the microorganism's ability to adhere to the tooth surface2,5,6,9. The potential decrease in bacterial metabolism and consequent reduction of adherence to the mucosa are properties attributed to this antimicrobial agent at a concentration of 0.05%, which is found in most commercial products. Cetylpyridinium chloride may also have increased efficacy if it is associated with other antimicrobial agents such as chlorhexidine solutions13.
Triclosan is a drug with proven power against bacterial plaque and it also has anti-inflammatory activity. It is a frequently used antiseptic with broad spectrum and low toxicity, has been used in cosmetology for over 20 years, is classified as a synthetic, anionic and liposoluble bisphenol1-2,5-6,8,10,14. It is more frequently used in the composition of the mouthwashes at a concentration of 0.03%15.
The action of this compound is based on the disruption of the plasma membrane by increasing its permeability and inhibiting the activity of trypsin-like enzymes, modifying the cellular transport and preventing adequate metabolism and reproduction of the bacterial cells, an event that does not appear to cause the imbalance of oral microflora4-6,14. It has a broad antimicrobial spectrum, acts against gram-positive and gram-negative bacteria, and it is effective against the mycobacterium genus, anaerobic bacteria, spores, and fungi of the Candida species2-3,5-6. However, because it is an agent with anionic charge, triclosan has low substantivity when incorporated into mouthwashes, so in order to remedy this deficiency, copolymers may commonly be associated with it. Gantrez is most frequently used, with the intention of enhancing the action of triclosan since increases its retention in the oral cavity, and consequently, its time of action. Therefore, its effectiveness is based on the increase in bioavailability, i.e. the possibility of keeping the concentration of this agent at the "diffusion boundary layer", found in the oral mucosa and on the tooth surface2,5,8,10,14. Due to the higher bioavailability, it may be affirmed that triclosan can reduce plaque by around 22% and gingivitis by 25%, this action is facilitated to an even greater extent by the low level of alcohol present in the formula providing pH stability of around 6.815.
In addition to the antimicrobial products mentioned, mouthwashes containing the essential oils thymol, menthol and eucalyptol are being sold with increasing frequency, and once they have been associated with the mouthwash formulation, these compounds exhibit antibacterial power. These substances are chemically classified as phenolic compounds, they have no charge, low substantivity and a high capacity of interaction with certain components in the bacterial plaque. The mechanism of action of these oils is based on their power to alter the healthiness of the cell wall particularly acting on Gram-positive bacteria and yeasts. Adverse effects such as a burning feeling and bitter taste may occur2-3,5-6.
When incorporated into mouthwashes containing antibacterial products, fluorides play an important role since they have the property of interfering in the dynamics of dental caries4,16. While the cariostatic action of fluoride results from the ability of this ion to interfere in the ecological balance of plaque, its protective action on enamel results from the rapid deposition on the tooth surface due to relatively low pH of these mouthwashes. When fluoride ion causes the inhibition of bacterial glycolytic enzymes present in human dental plaque, it promotes the blocking of carbohydrate metabolism, reduces bacterial acidogenesis and enamel demineralization. In parallel, it interferes in the biosynthesis of polysaccharides responsible for the adhesion of microorganisms onto the dental surfaces and contributes to enamel remineralization5,17. Finally, by knowing the composition of several mouthwashes, the concentrations of the active ingredients and their properties, dentists can choose the most suitable one for each clinical situation and guide patients correctly.
METHODS
The present research, classified as observational and descriptive study, was motivated by the frequent professional prescription of the most varied types of mouthwashes sold on the Brazilian market, parallel to the indiscriminate use of these products by the general population. Therefore, the aim of this study was to conduct a survey of the mouthwashes sold in Brazil, particularly in the city of Salvador, Bahia, in 2011, with the purpose of disseminating information about their components, pharmacological properties and indications. Therefore, the data were collected on the main mouthwashes, their various active ingredients, particularly antimicrobial agents, available commercially in that city, which corresponded to a sample of 29 products. This information was supplemented by the current scientific literature and data released on the internet by major manufacturers.
The results were organized with the purpose of precisely characterizing the various products analyzed, their compositions and concentrations, and comparing the different active antimicrobial ingredients of the mouthwashes. The other constituent elements in the formula, among them fluoride ions, were also taken into consideration. These data are descriptively presented and duly explained in the Tables.
RESULTS
To begin with, it must be pointed out that the results of this study may be generalized and therefore identified throughout Brazilian territory and in those countries that allow the sale of the active ingredients contained in the mouthwashes, bearing in mind that most of the original manufacturers of these mouthwashes are international companies.
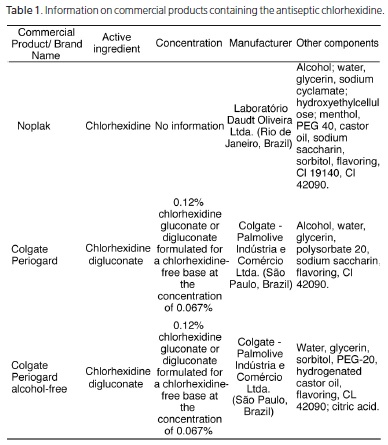
The information collected about the mouthwashes studied was organized in Tables 1, 2, 3 and 4. The following are antimicrobial active ingredients: cetylpyridinium chloride, triclosan, chlorhexidine and essential oils and their concentrations. In the analysis of the 29 mouthwashes evaluated, in addition to the active ingredient, the information related to the manufacturers and other components present in each formula were considered. Chart 1 shows the 3 mouthwashes whose active ingredient is chlorhexidine, also denominated as 0.12% chlorhexidine gluconate, or digluconate formulated for a chlorhexidinefree base at a concentration of 0.067%. A thorough evaluation revealed that two out of three mouthwashes evaluated - Colgate Periogard with and without alcohol produced by Colgate - Palmolive Industria e Comercio Ltda. (Sao Paulo, Brazil) discriminate the concentration of the active ingredient (0.12%) on the commercial label. The commercial product Noplak, a mouthwash manufactured by the laboratory Daudt Oliveira Ltda. (Rio de Janeiro, Brazil) was the only mouthwash that did not inform the concentration of the antimicrobial agent in its composition. It was also found that all three mouthwashes mentioned above have some humectant agent designed to provide viscosity, or similar colorings, flavorings, glycerin, sodium cyclamate and (or) sodium saccharin among other components (Table 1).
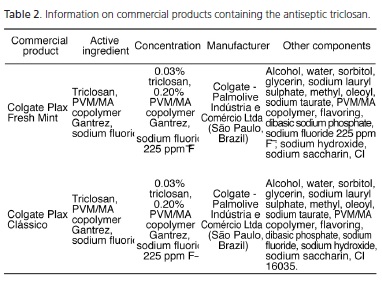
In Table 2 the mouthwashes that have triclosan as their active ingredient are grouped together. It was found that the number of commercially available mouthwashes is even smaller and the products sold are from the same manufacturer. The two mouthwashes are: Colgate Plax Fresh Mint and Colgate Plax Classic, associated with the PVM/MA copolymer Gantrez at a concentration of 0.2%. These mouthwashes contain the antimicrobial agent triclosan at a concentration of 0.03% and as previously mentioned they are produced by the same manufacturer, Colgate - Palmolive. Indústria e Comércio Ltda. (São Paulo, Brasil). It was also found that the two mouthwashes have the same composition, only being differentiated by the coloring used. Colgate Plax Fresh Mint uses the coloring CL 47005 and CL 42090 and Colgate Plax Clássico uses CL 16035.
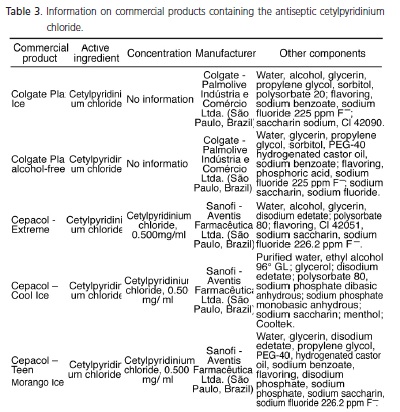
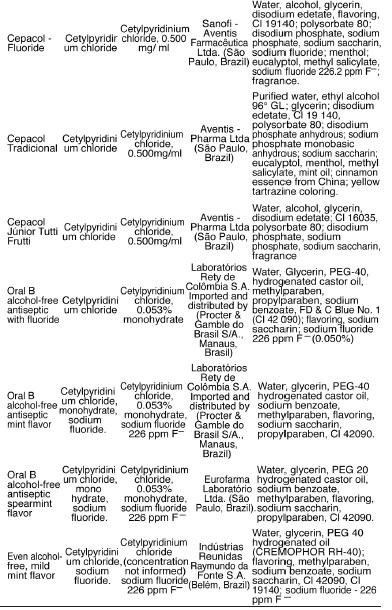
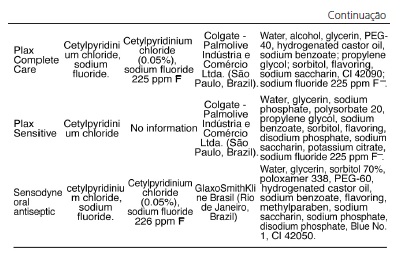
The mouthwashes containing cetylpyridinium chloride as the active ingredient are shown in Table 3 and this is an antiseptic substance that is present in the largest number of mouthwashes on the market, a total of 15 products. A large number of mouthwashes researched (11) state the concentration of the antimicrobial agent on their labels, whereas in 4 products, Colgate Plax Ice, Colgate Plax alcohol-free, Plax Sensitive (Colgate - Palmolive Industria e Comercio Ltda., São Paulo, Brazil), and Even alcohol-free mild mint flavor (Indústrias Reunidas Raymundo da Fonte SA, Belém, Brazil), the concentrations are not informed. Among the products that inform the concentration of the active ingredient on the label, six of them contain 0.500 mg of cetylpyridinium chloride per ml of mouthwash: Cepacol - Extreme, Cepacol Cool Ice, Cepacol - Teen Morango Ice, Cepacol - Fluoride (Sanofi - Aventis Farmacêutica Ltda., São Paulo, Brazil), Cepacol Tradicional, and Cepacol Júnior Tutti-Frutti (Aventis - Pharma Ltda., São Paulo, Brazil), while the others show variable concentrations of the agent that range from 0.050% to 0.053%: Oral B alcohol-free antiseptic with fluoride, Oral B alcohol-free antiseptic mint flavor, Oral B alcohol-free antiseptic spearmint flavor, Plax Complete Care, and Sensodyne oral antiseptic, the first two being distributed by Procter & Gamble of Brazil S/A., Manaus, Brazil, the third by Eurofarma Laboratório Ltda. (São Paulo, Brasil), the fourth by Colgate-Palmolive Indústria e Comércio Ltda. (São Paulo, Brazil) and the last one by GlaxoSmithKline Brasil (Rio de Janeiro, Brazil). It is worth noting that all products under the name Cepacol use sodium saccharin in place of sorbitol, a natural sweetener included in the composition of most mouthwashes on the market. In addition to having antimicrobial activity, the mouthwash Sensodyne is the only one with information on the label stating that it acts against tooth sensitivity due to the presence of sodium fluoride and sodium phosphate, and also contains sorbitol at a concentration of 70%.
Table 4 shows the distribution of the components of the 9 mouthwashes analyzed in this study, which contain essential oils as active ingredients in their formula. Seven of these mouthwashes associate thymol with menthol, eucalyptol and methyl salicylate, while the Listerine Whitening – Pre-brushing product (Johnson & Johnson Industrial Ltda., São Paulo, Brazil) contains only menthol associated with eucalyptol and Colgate Plax Whitening (Colgate - Palmolive Industria e Comercio Ltda. (São Paulo, Brasil) only contains menthol as the active ingredient. These two products contain hydrogen peroxide in their compositions; the mouthwash Listerine Whitening - Pre-brushing (Johnson & Johnson Industrial Ltda., São Paulo, Brazil) does not provide information about the concentration of the substance, while the concentration of this compound in Colgate Plax Whitening (Colgate - Palmolive Industria e Comercio Ltda., São Paulo, Brazil) is 1.5%. We must highlight the occurrence of sucralose in Listerine - Vanilla Mint (Pfizer Inc. - Lilitz, Pennsylvania, USA) and Listerine Total Care (Johnson & Johnson Industrial Ltda., São Paulo, Brazil) because it is a sweetener derived from the selective replacement of hydroxyl groups by chlorine at carbons 4 and 6 in sucrose. Its sweetening power is 600 times that of sugar, it has no calories, thermal and chemical stability, sweetness can vary 400-800 times in relation to sucrose and twice that of saccharin, and it is not cryogenic10.
It was also found that mouthwashes with similar names come from different manufacturers, such as Oral B alcohol-free antiseptic mint flavor, Oral B alcohol-free antiseptic spearmint flavor, Oral B with alcohol-free with fluoride are produced by the laboratory Rety Colombia SA (imported and distributed by Procter & Gamble of Brazil S/A., Manaus, Brazil) and Eurofarma Laboratório Ltda. (São Paulo, Brasil) respectively. It should also be placed on record that there is a significant difference among these these three mouthwashes, which is the incorporation of 226.2 ppm F- in the form of sodium fluoride in the product Oral B alcohol-free with fluoride (Procter & Gamble Brazil S/A., Manaus, Brazil). Finally, sodium fluoride may be present in other mouthwashes containing antibacterial active ingredients. The results obtained in this study show that when this ion is incorporated into the formula or when the concentrations are not explicit, or if they are present, the concentrations range between 220 and 226.2 ppm F-.
DISCUSSION
Analysis of the information presented on the labels of the main industrialized mouthwashes allowed them to be grouped according to their active ingredients, i.e. the antimicrobial agents cetylpyridinium chloride, triclosan, essential oils and chlorhexidine. Careful evaluation of the 29 mouthwashes enabled identification of the active ingredients, their concentration and complementary information about the other components in the formula, and the manufacturers.
The mouthwash containing 0.067% chlorhexidine (chlorhexidine digluconate or chlorhexidine gluconate) as the active ingredient are considered antimicrobial pharmachological products required by the population and are widely prescribed by professionals, according to several reports in the scientific literature. This drug has the power to promote the disruption of bacterial cell wall and inhibit enzymes present in the membrane and cytoplasm, hence it is considered the gold standard because of its proven effect of inhibiting plaque formation3,10. However, although this antimicrobial effect compromises the integrity of the vegetative forms of bacteria, the same effectiveness is not found against the sporulated microorganisms, except at high temperatures19. The frequent market demand for chlorhexidine solutions is attributed to the many features of this antibacterial compound, such as broad spectrum of antimicrobial activity, storage stability, lack of toxicity, substantivity, and relatively low cost11. Among the three mouthwashes assessed in the present study, only the mouthwash manufactured by the laboratory Daudt Oliveira Ltda. (Rio de Janeiro, Brazil), Noplak, did not inform the concentration of the active agent on its label (Table 1). The other mouthwashes studied informed the concentration of 0.12% chlorhexidine on their labels, irrespective of whether the vehicle was alcoholic or aqueous. It is also noted that these mouthwashes, as well as those based on cetylpyridinium chloride and triclosan, have glycerin in their formulae, a colorless, syrupy, liquid trialcohol that is extracted by saponification of fatty bodies. The trialcohol incorporated into these mouthwashes has a humectant function, with the purpose of maintaining viscosity, as it is hydrophilic and it helps to maintain moisture6,20-22. In addition to these components, chlorhexidine-based mouthwashes contain blue coloring CL 42090, a watersoluble compound.
As shown in Table 2, the mouthwashes on the market, with triclosan - a broad-spectrum antimicrobial agent with low toxicity - as the active ingredient, are those offering fewer choices, since they all come from the same industrial source. This restricts the consumers' option. Thus, only two mouthwashes are available: Colgate Plax Fresh Mint and Colgate Plax Classic (Colgate - Palmolive Indústria e Comércio Ltda., São Paulo, Brazil). Both associate 0.03% triclosan with the PVM/MA copolymer Gantrez at a concentration of 0.20%, in addition to sodium fluoride 225 ppm F-. This association is necessary due to the chemical characteristics of triclosan, which is an agent with anionic charge, with rapid dissolution. The addition of the copolymer increases the time of the antimicrobial agent permanence in the oral cavity, therefore, increasing its period of action2,8,10,24. The effectiveness of antiplaque activity and substantivity of 0.03% triclosan assessed in vivo are limited in the absence of Gantrez, which is the reason why the copolymer increases the spectrum of action of the antimicrobial component compromising the integrity of gram-negative and gram-positive bacteria and yeasts2,3,19. Finally, two mouthwashes are differentiated only by the coloring used: Colgate Plax Fresh Mint uses colorings CL 47005 and CL 42090 and Colgate Plax Clássico uses CL 16035, both manufactured by Colgate - Palmolive Indústria e Comércio Ltda. São Paulo, Brazil.
The oral anti-septics containing cetylpyridinium chloride are the most common on the market, totaling 15 products manufactured by different manufacturers. This antimicrobial agent acts primarily on Gram-positive bacteria and yeasts, producing increased permeability of the bacterial cell wall, thus favoring lysis and compromising cellular metabolism3,6,10,22. Among the 15 products, there is a lack of information on the concentration of the active ingredient in 4 of them: Colgate Plax Ice, Colgate Plax alcohol-free and Sensitive Plax manufactured by Colgate - Palmolive Indústria e Comércio Ltda. (São Paulo, Brazil); Even alcohol-free mild mint flavor under the responsability of Indústrias Reunidas Raymundo da Fonte S.A. (Belém, Brazil); and the following six at a concentration of 0.500 mg/ml: Cepacol - Extreme; Cepacol - Cool Ice; Cepacol - Teen Morango Ice; and Cepacol – Fluoride manufactured by Sanofi - Aventis Farmacêutica Ltda. (São Paulo, Brazil); and Cepacol Tradicional and Cepacol Júnior Tutti-Frutti manufactured by Aventis - Pharma Ltda. (São Paulo, Brasil). This other mouthwashes contain a concentration that ranges from 0.050% to 0.053% (Table 3). It was also found that mouthwashes with the same brand name come from different manufacturers, such as Oral B alcohol-free antiseptic mint flavor, Oral B alcohol-free antiseptic spearmint flavor produced by the laboratory Rety de Colombia SA (imported and distributed by Procter & Gamble of Brazil S/A., Manaus, Brazil) and Eurofarma Laboratório Ltda. (São Paulo, Brazil), respectively. Probably the latter company is responsible for the distribution of the mouthwash in Brazil, and its industrial control comes from the headquarters in Colombia. It is worth noting that cetylpyridinium chloride, commercially sold as Cepacol, is commercially distributed by Aventis - Pharma Ltda. (São Paulo, Brazil) and Sanofi-Aventis Ltda. (São Paulo, Brasil). The six presentations available on the market have the same concentration of the active product, ie 0.500 mg/ ml, and differentiated only by the "commercial name" and incorporation of sodium fluoride 226.2 ppm F- in the formula of three of them. Various substances are incorporated into different formula of Cepacol, for example cinnamon essence in China, hydrogenated castor oil, tartrazine yellow coloring (CL 19140), propylene glycol and even essential oils such as eucalyptol and menthol, the latter present in all of the formula of all products. It was also found that these products contain sodium saccharin sweetener in place of sorbitol, a natural sweetener in the composition of most mouthwashes (Table 3). In addition to having antimicrobial activity, In addition to having antimicrobial activity, the mouthwash Sensodyne (GlaxoSmithKline Brasil, Rio de Janeiro, Brazil) is the only one with information on the label stating that it acts against tooth sensitivity due to the presence of sodium fluoride and sodium phosphate, and also contains sorbitol at a concentration of 70%.
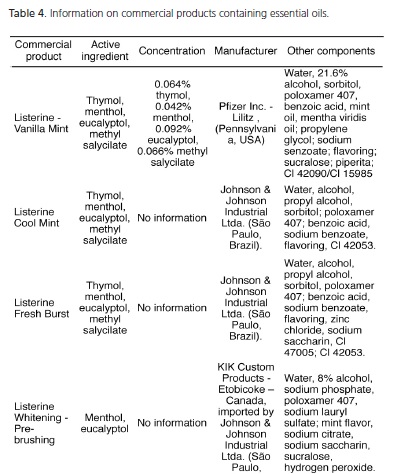
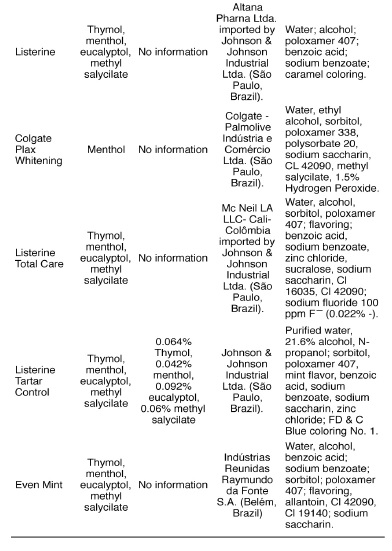
The essential oils present in the composition of 9 mouthwashes grouped together in Table 4 are thymol, menthol and eucalyptol reinforced by the addition of methyl salicylate. From a molecular point of view, these essential oils with antimicrobial effectiveness fall into the category of phenolic chemical compounds6. These active ingredients have a mechanism of action that causes changes in bacterial cell wall affecting mainly the gram-positive bacteria and yeasts3,24. Seven of these mouthwashes contain thymol, menthol, eucalyptol and methyl salicylate, while Listerine Whitening – Pre-brushing (Johnson & Johnson Industrial Ltda., São Paulo, Brazil) contains only menthol associated with eucalyptol and Colgate Plax Whitening (Colgate - Palmolive Industria e Comercio Ltda., São Paulo, Brazil) contains only menthol. Although the latter contains hydrogen peroxide in its composition at a concentration of 1.5%, the manufacturer does not explain if the function of this substance is to provide dental whitening. In view of this property, the whitening effectiveness, time of permanence in the oral cavity, number of daily mouthrinses and concentration of 1.5%, among other relevant issues, are questioned. It is also noted that some products have the same brand name, as in the case of Even alcohol-free mild mint flavor and Even mint, both manufactured by the the company (Indústrias Reunidas Raymundo da Fonte SA, Belém, Brazil), and several distinct components. The first one contains the active ingredient cetylpyridinium chloride associated with sodium fluoride 226 ppm F-, while the second one contains thymol, menthol, and methyl salicylate. It is worth noting that the concentrations of the antibacterial agents present in the mouthwashes are not informed. This observation is relevant because such distinction could only be made by careful analysis of the consumers of the product labels, since there is no discrimination of any kind on the front labels of these products.
The mouthwashes Listerine (Johnson & Johnson Industrial Ltda., São Paulo, Brazil) and Even mint (Indústrias Reunidas Raymundo da Fonte S.A., Belém, Brazil) contain poloxamer 407 in their composition, while Colgate Plax Whitening (Colgate - Palmolive Indústria e Comércio Ltda., São Paulo, Brazil) contains poloxamer 338 (Table 4). These are surfactant, non-ionic, hydrophilic compounds. Chemically they are trinucleated copolymers, i.e. they consist of a central block containing a hydrophobic fraction of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol. They have a surfactant action due to the property of promoting the dissolution of the other compounds in a certain solution. Listerine Whitening - Prebrushing (Johnson & Johnson Industrial Ltda., São Paulo, Brazil) and Colgate Plax Whitening (Colgate - Palmolive Industria e Comercio Ltda., São Paulo, Brazil) contain hydrogen peroxide in their composition, but there is no information recorded about the concentration of this substance. Of the 29 mouthwashes analyzed, only nine contain the sweetener sorbitol, and among the nine, seven have the various formulations of Listerine (Johnson & Johnson Industrial Ltda., São Paulo, Brazil).
One cannot lose sight of the concern about the lack of information on the concentration of all components that compose the mouthwashes, with the exception of the information with regard to the majority of the antibacterial agents. This concern grows due to the lack of information about the purposes and possible consequences that may result from the addition of these complements, for example ethyl alcohol and propyl alcohol. Finally, it is hoped that the data collected and evaluated in the present study may help dentists with the choice and possibile indications of mouthwashes in order to use them according to each clinical case, and that it may contribute to alert patients about the risks self-medication.
CONCLUSION
Based on the evaluation of the results obtained in this study, it may be concluded that there is wide variety of mouthwashes available on the Brazilian market, in particular in the city of Salvador, containing antimicrobial agents of different spectra, although cetylpyridinium chloride was the most frequently found substance. It was also found that irrespective of the active ingredient, the mouthwashes usually have the same complementary substances, especially the addition of fluoride ions, however, there is a limited amount of information available regarding the concentrations, indications and possible consequences that may result from their frequent use without professional supervision. Taking into account the diversity of mouthwashes, the different spectra of antibacterial action, and the unrestricted acquisition of these drugs, the benefits and adverse consequences of the indiscriminate use of these products should be widely disseminated, particularly among dentists.
Collaborators
DB ARAÚJO, EJ CAMPOS, IHA BASTOS, DM PAULA, ER TENÓRIO JUNIOR and RPC ARAÚJO participated in the preparation of the project, collection and interpretation of the data and in the writing of the article.
REFERENCES
1. Gomes EF, Passos IA, Verás Neto L, Padilha WWN. Atuação de três colutórios na condição de higiene e saúde bucal: estudo comparativo [citado 2010 Out 2]. Disponível em: <http://www.odontologia.com.br/artigos.asp?id=440> [ Links ].
2. Marinho BVS, Araújo ACS. O uso dos enxaguatórios bucais sobre a gengivite e o biofilme dental. Int J Dent. 2007;6(4):124- 31.
3. Bugno A, Nicolleti MA, Almodóvar AAB, Pereira TC, Auricchio MT. Enxaguatórios bucais: avaliação da eficácia antimicrobiana de produtos comercialmente disponíveis. Rev Inst Adolfo Lutz. 2006;65(1):40-5.
4. Nascimento AP, Faria G, Watanabe E, Ito IY. Efficacy of mouthrinse spray in inhibiting cariogenic biofilm formation on toothbrush bristles. Braz J Oral Sci. 2008;7(24):1989-92.
5. Torres CRG, Kubo CH, Anido AAnido, Rodrigues JR. Agentes antimicrobianos e seu potencial de uso na odontologia. PGR: Pós-Grad Rev Fac Odontol. 2000;3(2):43-52.
6. Moreira ACA, Pereira MHQ, Porto MR, Rocha LAP, Nascimento BC, Andrade PM. Avaliação in vitro da atividade antimicrobiana de antissépticos bucais. Rev Ciênc Méd Biol. 2009;8(2):153-61.
7. Dantas EM, Xavier CEA, Medeiros Segundo GX, Paiva MAPassos, Lima KLF. Pigmentação dentária por clorexidina: relato de caso clínico. Odontol Clín- Científ. 2007;6(2):175-8.
8. Pires JR, Rossa Junior C, Pizzolitto AC. In vitro antimicrobial efficiency of a mouthwash containing triclosan/gantrez and sodium bicarbonate. Braz Oral Res. 2007;21(4):342-7. doi: 10.1590/S1806-83242007000400011.
9. Semenoff TADV, Semenoff-Segundo A, Biasoli ER. Efetividade antimicrobiana in vitro de enxaguatórios bucais frente aos microorganismos Staphylococcus aureus e Pseudomonas aeroginosa. Rev Odonto Ciência. 2008;23(4):351-4.
10. Segundo AS, Bosco IF, Semenoff TADV, Rocatto GEGD, Cirilo DM, Buzelle SL, Nunes SO. Efetividade do gluconato de clorexidina a 0,12% e do digluconato de clorexidina a 2% adquiridos em diferentes dentais e farmácias na cidade de Cuiabá, sobre cândida albicans. Periodontia. 2007;17(1):41-5.
11. Bambace AMJ, Barros EJA, Santos SSF, Jorge AOC. Eficácia de soluções aquosas de clorexidina para a desinfecção de superfícies. Rev Biociênc. 2003;9(2):73-81.
12. Baptista PCS, Araújo AN, Montenegro MCBSM. Determinação potenciométrica em fluxo de cloreto de cetilpiridínio em desinfectantes bucais. Quím Nova. 2003;26(4):475-8. doi: 10.1590/S0100-40422003000400005.
13. Yévenes I, Alvarez SR, Jará MN, Wolfenson PM, Smith LP. Comparison of mouthrinses containing chlorhexidine and other active agents with chlorhexidine mouthrinse-gel: effects on de novo plaque formation. Rev Odonto Ciênc. 2009;24(4): 345-8.
14. Nogueira-Filho GR, Toledo S, Cury JA. Avaliação do efeito de um dentifrício contendo triclosan-gantrez - zinco-pirofosfato na gengivite experimental em humanos. Periodontia. 1997;6(Supl.):20-4.
15. Zanin SMW. Enxagautório bucal: princípios ativos e desenvolvimento de fórmula contendo extrato hidroalcoólico de Salvia officinalis L. Visão Acad. 2007;8(1):19-24.
16. Moi GP, Tenuta LMA, Cury JA. Anticaries potential of a fluoride mouthrinse evaluated in vitro by validated protocols. Braz Dent J. 2008;19(2):91-6. doi: 10.1590/S0103-64402008000200001.
17. Lima AL, Valença AMG, Albuquerque FR, Silva NB. Análise do pH e da viscosidade de enxaguatórios bucais fluoretados disponíveis comercialmente na cidade de João Pessoa-PB. Pesq Bras Odontoped Clin Integr. 2005;5(3):223-8.
18. Torloni MR, NakamuraII MU, Megale A, Sanchez VHS, Mano C, Fusaro AS, Mattar R. O uso de adoçantes na gravidez: uma análise dos produtos disponíveis no Brasil. Rev Bras Ginecol Obstet. 2007;29(5):267-75. doi: 10.1590/S0100- 72032007000500008.
19. Siqueira JF Jr, da Silva CH, Cerqueira M das D, Lopes HP, de Uzeda M. Effectiveness of four chemical solution in eliminating Bacillus subtilis spores on gutta-percha cones. Endod Dent Traumatol. 1998;14(3):124-6.
20. Prista LN, Bahia MF, Vilar E. Dermofarmácia e cosmética. Porto: Associação Nacional de Farmácia; 1995.
21. Simões CSS, Merlini S, Silva RPR, Bastos RS, Torres AS, Bastos JRM. Avaliação in vitro da atividade antimicrobiana de enxaguatórios bucais. Rev Bras Odontol. 2011;68(1):91-4.
22. Borges AH, Pedro FLM, Semenoff TDS, Porto NA, Segundo AS, Buzelle SL. Efetividade antimicrobiana de diferentes marcas comerciais de enxaguatórios bucais com e sem álcool sobre diversos microrganismos: estudo in vitro. Rev Odonto Ciênc. 2010;25(2):178-81. doi: 10.1590/S1980-65232010000200014.
23. Maekawa LE, Brighenti FL, Lamping R, Oliveira LD, Marcacci S, Koga-Ito CY. Atividade antimicrobiana de enxaguatórios bucais sem álcool à base de clorexidina sobre Candida albicans. Rev Odontol UNESP. 2010;39(1):15-9.
24. André RF, Andrade IM, Silva-Lovato CH, Paranhos Hde F, Pimenta FC, Ito IY. Prevalence of mutans streptococci isolated from complete dentures and their susceptibility to mouthrinses. Braz Dent J. 2011;22(1):62-7. doi: 10.1590/S0103- 64402011000100011.
 Correspondence to:
Correspondence to:
DB ARAUJO
e-mail: danilobarral81@hotmail.com
Received on: 14/6/2011
Final version resubmitted on: 14/9/2011
Approved on: 17/10/2011













