RGO.Revista Gaúcha de Odontologia (Online)
ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.4 Porto Alegre oct./dic. 2012
ORIGINAL / ORIGINAL
Biocompatibility analysis of a novel reabsorbable alloplastic membrane composed of alginate-Capsul
Análise da biocompatibilidade de uma membrana aloplástica reabsorvível composta de alginato-capsul
Cristina JARDELINO I; Igor Iuco CASTRO-SILVA II; Callinca Paolla Gomes MACHADO I; Maria Helena ROCHA-LEÃO III; Alexandre Malta ROSSI IV; Silvia Rachel de Albuquerque SANTOS IV; José Mauro GRANJEIRO V
I Universidade Federal Fluminense, Hospital Universitário Antônio Pedro, Unidade de Pesquisa Clínica. Niterói, RJ, Brasil
II Universidade Federal Fluminense, Programa de Pós-Graduação em Odontologia. Niterói, RJ, Brasil
III Universidade Federal do Rio de Janeiro, Escola de Química. Rio de Janeiro, RJ, Brasil
IV Centro Brasileiro de Pesquisas Físicas, Laboratório de Biomateriais. Rio de Janeiro, RJ, Brasil
V Instituto Nacional de Metrologia, Normalização e Qualidade Industrial - INMETRO. Duque de Caxias, RJ, Brasil
ABSTRACT
Objective
The aim of this study was to evaluate in vivo the biological response after implantation of a novel alginate-capsule membrane.
Methods
The material was implanted into subcutaneous tissue of mice (n=15) and after 1, 3 and 9 weeks, the animals were sacrificed and biopsies analyzed with light microscopy, using the stains hematoxylin-eosin, picrosirius and alcian blue pH 2.5. The parameters evaluated were: intensity and kind of inflammatory infiltrate, presence of connective tissue, foreign body reaction, vascularization and biodegradation.
Results
1 week after implantation, the following was observed: mixed inflammatory infiltrate, absence of necrosis and beginnings of membrane fragmentation; after 3 weeks, discrete presence of multinuclear giant cells and beginnings of neovascularization; and after 9 weeks there was minor biodegradation associated with the presence of new connective tissue, and persistence of moderate inflammatory reaction observed from beginning to end of the experiment.
Conclusion
Considering the results obtained, it is possible to conclude that the novel alginate-capsule membrane is partially reabsorbable but with low biocompatibility, requiring more tests to validate its clinical use.
Indexing terms: Material testing. Seaweed. Tissue engineering.
RESUMO
Objetivo
Avaliar in vivo a resposta tecidual após a implantação de uma nova membrana de alginato-capsul.
Métodos
O material foi implantado no tecido subcutâneo de camundongos (n=15) e após 1, 3 e 9 semanas, os animais foram mortos e as biópsias analisadas à microscopia de luz, através de coloração com hematoxilina-eosina, picrosirius e azul de alcian pH 2,5. Os parâmetros avaliados foram: intensidade e tipo de infiltrado inflamatório, presença de tecido conjuntivo, reação de corpo estranho, vascularização e biodegradação.
Resultados
Após 1 semana da implantação, notou-se infiltrado inflamatório misto, ausência de necrose e início de fragmentação da membrana, em 3 semanas, observou-se presença discreta de células gigantes multinucleadas e início de neovascularização, e em 9 semanas houve pequena biodegradação associada com a presença de novo tecido conjuntivo e persistência de reação inflamatória moderada observada desde o início do experimento.
Conclusão
Considerando os resultados obtidos concluiu-se que a nova membrana de alginato-capsul é parcialmente reabsorvível, mas com baixa biocompatibilidade, necessitando de mais testes para validar seu uso clínico.
Termos de indexação: Teste de materiais. Alga marinha. Engenharia tecidual.
INTRODUCTION
The concepts of Guided Tissue Regeneration (GTR) and Guided Bone Regeneration (GBR) were first described in the literature in 19591-2. Both are based on four basic principles: biocompatibility, maintenance of adequate space to support the newly formed tissue, the promotion of growth of osteogenic cells and peripheral sealing to prevent growth of unwanted cellular tissue inside the defective area and the displacement of the membrane3-5. In this context, the development of new membranes that will serve these principles and permit safe, large-scale and low-cost production, has been a challenge for tissue engineering.
Alloplastic materials are mineral or synthetic in origin and, as they result from a process of industrialization, they can be supplied in adequate quantities for all needs. Due to the smaller osteoconductive capacity when compared to autogenic bone, as well as being osteoinductive and osteogenic, these materials may have different chemical formulations and associations with the strategy of improving performance6.
Sodium alginate, which derives from brown algae found in the oceans, is a linear polysaccharide formed by units of β-D-mannuronic acid (M) and α-Lguluronic acid (G) linked by type 1-4 glycosidic bonds, forming alginic acids. The proportion between the monomeric units is variable, as is the G or M sequence of units in the polymer chain. It is used as a thickener and emulsion stabilizer in the foods industry and as a modeler in Dentistry. It forms a reticulate gel in the presence of divalent and trivalent cations, the calcium alginate having a high incidence of G sequences (block G) being mostly used for capturing live cells and bioactive substances7. The action mechanism of the alginates is based on an ion exchange reaction between the calcium ions of the biomaterial and sodium ions present in the ooze of the wound, allowing the alginate fibers to be transformed into a soluble gel that promotes a damp microenvironment in the injury which is ideal for the process of healing8-10.
Capsul, also known as octenylsuccinate starch is obtained through the esterification of waxy starch with the acid anhydrous octenylsuccinate, which results in a hydrophobically modified starch11, capable of improving the texture of pastes or gels and the formation of film, adding hydrophobic groupings and introducing emulsifying power12.
Starch and alginate are polymers with an ability to capture molecules in technologies for the controlled release of bioactive substances13. A mixture of alginate: Capsul (3:4) produced edible film with adequate capture properties and vitamin C protection for 90 days7-14.
Therefore the aim of the present study is to analyze the biocompatibility and biodegradability of a new alginate- Capsul alloplastic membrane under the skin of mice.
METHODS
The test material used in this study was an alloplastic membrane created from alginate (Fluka, USA) and Capsul (National Starch & Chemical, USA), in a proportion of 3:4, and then treated via immersion in a solution of calcium chloride (0.15M CaCl2), and adjusted to dimensions of 5mm x 5mm for the biocompatibility trial (Figure 1). Prior to carrying out the experiment, each material was individually packed and underwent damp heat sterilization in an autoclave (Cristófoli, Brazil), temperature=127ºC, pressure=1atm, cycle time=20 minutes.
The biological characterization (biocompatibility and biodegradation of the membrane) was conducted in accordance with the procedures of ISO standard 10993- 6 (International Organization for Standardization)15. This study was approved by the Ethics in Research Committee of the Fluminense Federal University (Opinion CEPA-UFF 35/08). All of the surgical procedures were carried out in the Experimental Surgery Laboratory of the Animal Facility at the Fluminense Federal University following the directives of the Brazilian College of Animal Experimentation. A total of 15 BALB/c mice were used, all 2 months old and weighing around 75g, kept in proper conditions of hygiene and overall care for the duration of the experiments.
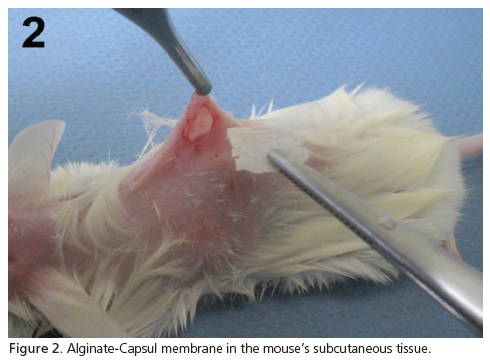
The animals received intramuscular anesthetic using a solution composed of ketamine chlorhydrate (Dopalen®, Vetbrands, Brazil; 100 mg/kg of animal) and xylazine chlorohydrate (Anasedan®, Vetbrands, Brazil; 10 mg/kg of animal). The aforementioned dosage of anesthetic produced the desired effects (immobilization and pain suppression of the specimen) and for the period of time required to carry out the surgical activity (30 minutes). After a trichotomy and dorsal antisepsis, a 1.5cm linear incision was carried out on each animal followed by dissection of the receptor region, implantation of the biomaterial into the subcutaneous tissue (Figure 2) and suture with 5.0 nylon thread (Ethicon®, Johnson & Johnson, Brazil). After 1, 3 and 9 weeks, the animals were put down using an anesthetic overdose and a necropsy was performed on the site of the implantation. The histological processing of the collected samples involved: fixation in 4% buffered formaldehyde (pH 7.2) for 48 hours; dehydration in increasing ethanol baths (1 bath at 70%, 80% and 90% and 3 baths at 100%, 1 hour each); diaphanization in xylene (3 baths, 30 minutes each), impregnation in liquid paraffin at 60°C (3 baths, 1 hour each); and inclusion in paraffin. Cuts 5μm thick were colored using the techniques of Hematoxylin-Eosin (HE), picrosirius and alcian blue pH 2.5 and they were submitted for descriptive analysis, which evaluated the following parameters: inflammatory reaction (presence and intensity of polymorphonuclear, mononuclear and multinucleated foreign body giant cells), repair process (granulation tissue, neovascularization, fibrosis) and biodegradation (presence or fragmentation of material).
RESULTS
Biocompatibility
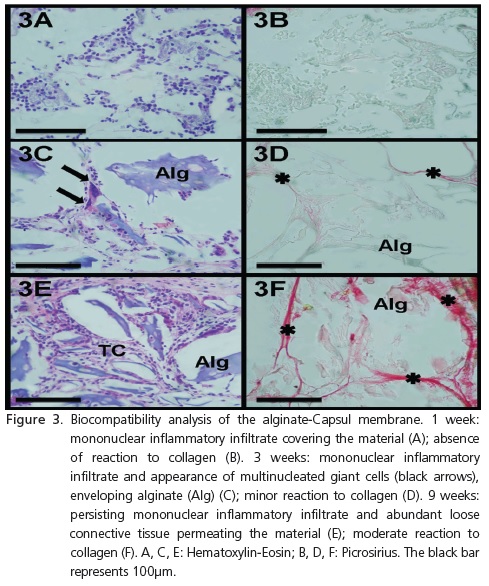
1 week - Presence observed of mixed inflammatory infiltrate (polymorpho- and mononuclear cells) of slight to moderate intensity, absence of areas of necrosis, absence of multinucleated foreign body giant cells (HE) (Figure 3A) and absence of reaction to collagen (picrosirius) (Figure 3B).
3 weeks - Presence of predominantly mononuclear inflammatory infiltrate, of slight to moderate intensity, presence of connective tissue permeating the material, discrete presence of new blood vessels and multinucleated foreign body giant cells enveloping the alginate (HE) (Figure 3C). The permeability of connective tissue is confirmed by the slight reaction to collagen (picrosirius) (Figure 3D).
9 weeks - Continuity of the mononuclear inflammatory infiltrate, of slight to moderate intensity, presence of loose connective tissue permeating the material in more significant quantities, with discrete vascularization and absence of multinucleated foreign body giant cells (HE) (Figure 3E). There is moderate reaction to collagen (picrosirius) (Figure 3F).
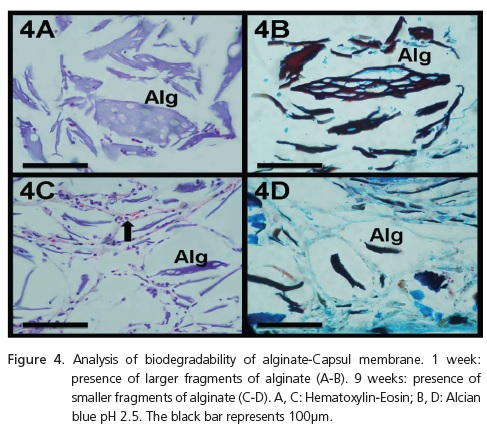
Biodegradability
1 week - The alginate matrix already shows the beginnings of degradation, with more sizeable fragments, greater than 100μm in extension (HE and alcian blue pH 2.5) (Figures 4A and 4B).
9 weeks - The alginate matrix shows process of more advanced degradation, with small fragments, less than 100μm in extension (HE and alcian blue pH 2.5) (Figures 4C and 4D).

DISCUSSION
Alginate is a natural polysaccharide whose biological behavior is being increasingly analyzed through tissue engineering. For many years it has been recognized as a biomaterial capable of causing inflammatory reaction. By itself, the surgical procedure for implantation of a material is capable of initiating an inflammatory response, however when in contact with the tissue, alginate induces macrophage activation16.
The characterization of the chemical structure of the alginate is still insufficient, however it is known that it presents lipopolysaccharide, which is a powerful activator of innate immune response17. An important regulator, of both innate immune response and adaptive immune response, recognized as a nuclear transcription factor (NFkB), it is activated when the organism comes into contact with alginate, stimulating macrophages to produce proinflammatory cytokines such as IL-1β, IL-6, IL-12 and TNF-a18-19.
The in vivo implantation of unpurified alginate is shown to be favorable, and is capable of stimulating cell proliferation20. In smaller bone defects in rabbits, an alginate membrane compared with a collagen showed double the bone neoformation (in 8 weeks) and degradation that was twice as fast (18 to 28 days and 28 to 56 days, respectively) in mandibular defects ( =5mm)21, and a greater presence of osteoinductive factors (VEGF, TGF-bβ and BMP) in defects of the tibia ( =5mm)22. Associating alginate with other components, such as hydroxyapatite, may have a positive influence on bone and vascular neoformation where there is extensive bone loss, as observed in skull defects in rats ( =8mm) in later periods (120 days)20.
Control over the degradability of the biomaterial is frequently a key factor in tissue replacement, since it will serve as the skeleton for growth and the integration of the new tissue23. The quantity and quality of new tissue to be formed are directly related to the speed of alginate degradation. Accordingly, gels with very fast degradation (100% volume loss in 5 days) prevent the maintenance of the tissue skeleton, while gels with moderate to slow degradation (less than 20% in 4 weeks) favor the proliferation of osteoblasts and stimulate the production of mineralized matrix24.
CONCLUSION
According to the results presented, the alginate- Capsul membrane presents favorable biodegradation and is capable of acting as a skeleton for cell proliferation and tissue neoformation. However, studies should be performed with a view to minimizing or controlling the intensity of the chronic inflammatory process triggered by its implantation. Modifications in its processing and purification, in addition to more pre-clinical studies, may contribute to the development of the material.
ACKNOWLEDGEMENTS
We would like to thank the research support Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (CNE Public Notice 2006), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Public Notice 24 Science & Technology-Health 2004) and the Financiadora de Estudos e Projetos (FINEP) (PPSUS Public Notice 2006) for their financial support.
Collaborators
C JARDELINO contributed with experimental surgery, histological processing, histological colorations, data interpretation and the composition of the article. II CASTROSILVA contributed with the histopathological analyses and the composition of the article. CPG MACHADO contributed with experimental surgery and the composition of the article. MH ROCHA-LEÃO, AM ROSSI and SRA SANTOS contributed to the development and production of test material and the composition of the article. JM GRANJEIRO contributed to the development of the test material, final interpretation of the data and the composition of the article.
REFERENCES
1. Dahlin C, Sennerby L, Lekholm U, Linde A, Nyman S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989;4(1):19-25. [ Links ]
2. Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290-6.
3. Aslan M, Simsek G, Dayi E. Guided bone regeneration (GBR) on healing bone defects: a histological study in rabbits. J Contemp Dent Pract. 2004;15:5(2):114-23.
4. Lundgren A, Lundgren D, Taylor A. Influence of barrier occlusiveness on guided bone augmentation. An experimental study in the rat. Clin Oral Implants Res. 1998;9(4):251-60. doi: 10.1034/j.1600-0501.1998.090406.x.
5. Oh TJ, Meraw SJ, Lee EJ, Giannobile WV, Wang HL. Comparative analyses of implant dehiscence defects. Clin Oral Impl Res. 2003;14(1):80-90.
6. Mah J, Hung J, Wang J, Salih E. The efficacy of various alloplastic bone grafts on the healing of rat calvarial defects. Eur J Orthod. 2004;26(5):475-82. doi: 10.1093/ejo/26.5.475.
7. Bastos D da S, Araújo KG, Leão MH. Ascorbic acid retaining using a new calcium alginate capsul based edible film. J Microencapsul. 2009;26(2):97-103. doi: 10.1080/02652040802175805.
8. Morgan D. Alginate dressings. J Tissue Viability. 1996;7(1):4-14.
9. Thomas S. Alginate dressings in surgery and wound management - Part 1. J Wound Care. 2000;9(2):56-60.
10. Thomas S. Alginates: a guide to the properties and uses of the different alginate dressings available today. J Wound Care. 1992;1(1):29-32.
11. Maia LH, Porte A, Souza VF. Filmes comestíveis: aspectos gerais, propriedades de barreira a umidade e oxigênio. Bol Centro Pesqui Process Aliment. 2000;16(1):104-28.
12. Silva GO, Takizawa FF, Pedroso RA, Franco CML, Leonel M, Sarmento SBS, et al. Características físico-químicas de amidos modificados de grau alimentício comercializados no Brasil. Ciênc Tecnol Aliment. 2006;26(1):188-97.
13. Shahidi F, Han XO. Encapsulation of food ingredients. Crit Rev Food Sci Nutr. 1993;33(6):501-47. doi: 10.1080/10408399309527645.
14. Bastos DS, Leão MHR, Araújo KGL, inventor; National Institute for Industrial Property, assignee. Filme polimérico comestível, método de preparo e uso. Brasil. Patente industrial n. 0701289-6.
15. ISO 10993. Biological evaluation of medical devices. Part 6: tests for local effects after implantation. Geneva: International Organization for Standardization; 2007.
16. Anderson JM. Mechanisms of inflammation and infection with implanted devices. Cardiovasc Pathol. 1993; 2(3):S33-S41. doi: 10.1016/1054-8807(93)90045-4.
17. Smetana K Jr. Cell biology of hydrogels. Biomaterials. 1993;14(14):1046-50. doi: 10.1016/0142-9612(93)90203-E.
18. Delhalle S, Blasius R, Dicato M, Diederich M. A Beginner's Guide to NF-ĸB Signaling Pathways. Ann N Y Acad Sci. 2004;1030(1):1- 13. doi: 10.1196/annals.1329.002.
19. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-ĸB and IĸB proteins: Implications in cancer and inflammation. Trends Biochem Sci. 2005;30(1):43-52. doi: 10.1016/j. tibs.2004.11.009.
20. De Paula FL, Barreto IC, Rocha-Leão MH, Borojevic R, Rossi AM, Rosa FP, et al. Hydroxyapatite-alginate biocomposite promotes bone mineralization in different length scales in vivo. Front Mater Sci China. 2009;3(2):145-53. doi: 10.1007/s11706-009- 0029-9.
21. Jianqi H, Hong H, Lieping S, Genghua G. Comparison of calcium alginate film with collagen membrane for guided bone regeneration in mandibular defects in rabbits. J Oral Maxillofac Surg. 2002;60(12):1449-54. doi: 10.1053/joms.2002.36108.
22. He H, Huang J, Chen G, Dong Y. Application of a new bioresorbable film to guided bone regeneration in tibia defect model of the rabbits. J Biomed Mater Res A. 2007;82(1):256-62. doi: 10.1002/jbm.a.31176.
23. Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, et al. Silk implants for the healing of critical size bone defects. Bone. 2005;37(5):688-98. doi: 10.1016/j.bone.2005.06.010.
24. Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 2001;56(2):228-33. doi: 10.1002/1097- 4636(200108)56:2<228:: AID-JBM1089>3.0.CO;2-9.
 Correspondence to:
Correspondence to:
JM GRANJEIRO
e-mail: jmgranjeiro@gmail.com
Received on: 13/11/2009
Approved on: 24/8/2010