Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.4 Porto Alegre Out./Dez. 2012
ORIGINAL / ORIGINAL
Resilon and Gutta-percha cones: an evaluation of biocompatibility in the connective tissue of rats
Avaliação da biocompatibilidade de cones de Resilon e de gutapercha em tecido conjuntivo de ratos
Fabiana Soares GRECCA I; Patrícia Maria Poli KOPPER I; Régis Burmeister dos SANTOS I; Anna Christina FOSSATI I; Vinicius Coelho CARRARD I; Gerson Arison Xavier ACASIGUA I; José Antônio Poli de FIGUEIREDO II
I Universidade Federal do Rio Grande do Sul, Faculdade de Odontologia, Departamento de Odontologia Conservadora. Porto Alegre, RS, Brasil
II Pontifícia Universidade Católica do Rio Grande do Sul, Faculdade de Odontologia. Porto Alegre, RS, Brasil
ABSTRACT
Objective
This study tested tissue response to gutta-percha or Resilon cones at 7, 14, 30, 60 and 90 days
Methods
Thirty Wistar rats each received three subcutaneous implants of polyethylene tubes containing: GP (gutta-percha); R (Resilon); CG (control group – empty tube). After histological processing, sections were analyzed by a calibrated examiner, using a light microscope to identify the presence of neutrophils, lymphocytes and plasma cells, eosinophils, macrophages and giant cells, fibrous capsule and abscesses, by an examiner using a light microscope. Kruskal-Wallis and multiple comparison tests were used for the statistical analysis.
Results
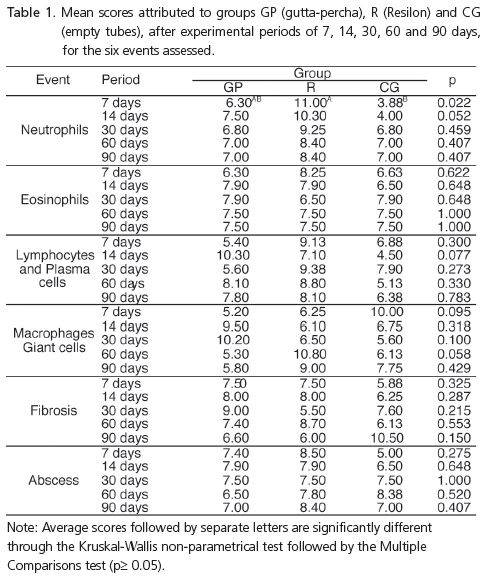
There were no differences for the macrophages and giant cells, lymphocyte and plasma cell infiltrate, eosinophils, fibrosis or abscesses. Higher neutrophil infiltrate scores were observed at 7 days for groups R and CG (p≤ 0.05). In group R, the number of neutrophils decreased with time (p= 0.017).
Conclusion
The results of the present study demonstrate that Resilon cones are well tolerated by tissues and have acceptable biocompatibility, in the same way as gutta-percha cones.
Indexing terms: Connective tissue. Endodontics. Root canal filling materials.
RESUMO
Objetivo
Avaliar a biocompatibilidade de cones de guta-percha e Resilon em tecido conjuntivo de ratos nos períodos de 7, 14, 30, 60 e 90 dias.
Métodos
Trinta ratos Wistar receberam três implantes de tubos de polietileno contendo: GP (guta-percha), R (Resilon), CG (grupo controle - tubo vazio). Após cada período experimental, foi realizado o processamento histológico e os cortes foram analisados por um examinador calibrado, com auxílio de microscópio de luz para identificar a presença de neutrófilos; linfócitos e células plasmáticas; eosinófilos, macrófagos e células gigantes; cápsula fibrosa e abscessos. Para a análise estatística foram utilizados os testes de Kruskal-Wallis e de comparações múltiplas.
Resultados
Não houve diferença para os macrófagos e células gigantes, linfócitos e células plasmáticas; eosinófilos, fibrose ou abscesso. Foram observadas altas taxas de infiltrado neutrofílico aos sete dias nos grupos R e GC (p ≤ 0,05). No grupo R, o número de neutrófilos diminuiu com o tempo (p = 0,017).
Conclusão
Os resultados do estudo demonstram que os cones de Resilon são bem tolerados pelos tecidos e possuem biocompatibilidade aceitável, como cones de guta-percha.
Termos de indexação: Tecido conjuntivo. Endodontia. Materiais restauradores do canal radicular.
INTRODUCTION
Root canal filling is one of the final stages of endodontic treatment. Materials used during filling should meet a number of criteria in order to stimulate apical and periapical repair after the filling procedure. Biocompatibility is one of these criteria, and is fundamental since the materials remain in close contact with periapical tissue.
Gutta-percha is the most widely employed solid filling material and it is supplied in the form of cones which are composed of organic (gutta-percha polymers, waxes and resins) and inorganic ingredients (zinc oxide and barium sulfate)1. However, thermoplastic cones, made from synthetic polymers (Resilon cones - RealSeal®, SybronEndo, Orange, CA, USA), have now been developed. According to Shipper et al.2, the advantage of Resilon cones is their excellent adhesion to resin-based sealers. According to the manufacturer, they are composed of a synthetic polymer (polyester), bioactive glass, bismuth oxychloride, barium sulfate and a dye.
The properties of gutta-percha are well documented and studies of its biocompatibility have shown good results3-8. Resilon cones, being a newer material, have not been fully studied. Onay et al.9 and Bodrumlu et al.10 found that the connective tissue of rats responded similarly to implants of gutta-percha and Resilon cones. These authors ascertained that the responses to both materials were good and both materials were considered to be biocompatible.
The subcutaneous implantation of materials is considered to be a suitable secondary test for the evaluation of the biocompatibility properties of restorative and endodontic materials11-16.
To determine the biocompatibility of Resilon cones, the aim of this in vivo study was to evaluate the tissue response to gutta-percha or Resilon cones in polyethylene tubes implanted in the subcutaneous connective tissue of rats at 7, 14, 30, 60 and 90 days.
METHODS
Thirty animals (Rattus norvegicus albinus Wistar) were grouped according to the 5 experimental periods (n=6): 7, 14, 30, 60 and 90 days. Inflammatory reactions were evaluated for 3 groups (n=30): GP - gutta-percha cones (Dentsply, Maillefer, USA); R - Resilon cones (SybronEndo™, Sybron Dental Specialties Inc., USA); CG - control group (empty tube).
Animals were anesthetized with 0.008 mL/100 g of ketamine and 0.004 mL/100 g of xylazine hydrochloride 2% (Virbac do Brasil Indústria e Comércio Ltda., São Paulo, SP, Brazil). The trichotomy of the dorsal region was carried out manually and the area was disinfected with alcoholiodine solution. Three 0.5 cm long incisions were made on each animal's back, 2 cm from the spine and at least 2 cm apart. Lateral tearing of the subcutaneous tissue allowed for 3 surgical cavities, equidistant from the center of the animal's back.
Ninety polyethylene tubes approximately 10 mm long and 1.5 mm in diameter (Abbott Lab do Brasil, São Paulo, SP, Brazil) were autoclaved. Gutta-percha (n=30) and Resilon (n=30) cones were carefully introduced into the tubes and sectioned to a length of 10 mm from the smaller-caliber end using a #15 scalpel blade (Becton Dickinson Indústrias Cirúrgicas Ltda., Juiz de Fora, Brazil) attached to a scalpel handle (Duflex™, SS White Artigos Dentários Ltda., Rio de Janeiro, Brazil).
The tubes were inserted into the surgical cavities, parallel to the incisions. Each animal received one tube from each group (GP, R, CG). The position in which the tubes of each group were implanted was standardized. Incisions were sutured with a 3-0 silk thread (Johnson & Johnson Produtos Profissionais Ltda., São José dos Campos, SP, Brazil).
At the end of each experimental period, 6 animals were anesthetized and sacrificed by means of cervical dislocation. Removal of the implant area was carried out using a safety margin of 1 cm, and the resulting specimens were fixed in formalin 10% for 24 hours. The tubes were removed from the specimens, which were then set in paraffin blocks and coded. Sections with a thickness of 5-6 μm were taken along the axis of the tube, mounted on slides and stained with hematoxylin-eosin. Three sections were evaluated per sample. The slides were examined under a light microscope at magnifications of 100x, 200x and 400x by a blinded examiner, according to the criteria described by Figueiredo et al.17. The examiner was calibrated before the data were analyzed.
The cellular inflammatory component was determined by the presence of neutrophils, lymphocytes/ plasmocytes, eosinophils, macrophages and giant cells; it was then classified according to the following scores: (1) absent (cells absent or inside vessels); (2) mild (cells present, but sparse or in reduced clusters); (3) moderate (cells present, yet not dominating the microscopic field); and (4) intense (cells present in the form of infiltrate).
Fibrous tissue was classified according to the following scores: (1) absence of collagen fibers; (2) presence of a thin layer of collagen fibers; and (3) presence of a thick layer of collagen fibers. Abscess formation was classified as follows: (1) absence of abscess; (2) presence of abscess in contact with the surgical cavity where the material had been; and (3) presence of abscess areas away from the surgical cavity where the material had been.
The non-parametric Kruskal-Wallis test was used to analyze the data statistically, and the multiple comparisons test to determine differences. The level of significance was set at α=0.05.
This study was structured according to the ethical principles contained in the Declaration of Helsinki (2000) and COBEA (Brazilian College of Animal Experimentation). The surgical procedures described here follow the incorporated and widely disseminated technical principles. This study was approved by the Institutional Review Board and by the Research Ethics Committee of the School of Dentistry of the Federal University of Rio Grande do Sul, Brazil, filed under record no. 12/07.
RESULTS
Some specimens were lost during histological analysis. Therefore, the final sample had 5 specimens in GP, and 4 in groups R and CG at 7 days. At 15 days, there were 5 in groups GP and R, and 4 in CG; at 30 days, 5 in groups GP and CG, and 4 in group R; at 60 and 90 days, 5 in groups GP and R, and 4 in group CG.
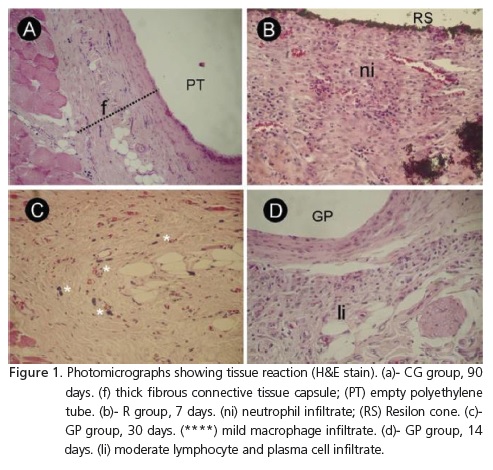
Figure 1 and Table 1 illustrate the behavior of the groups in terms of the events assessed, in each observation period. It was observed that the groups did not exhibit differences over time for the presence of macrophages and giant cells, lymphocyte and plasma cell infiltrates, eosinophils, fibrous tissue or abscesses (p≥0.05). Neutrophil infiltrate scores were significantly higher for group R than those for the control group at 7 days (p=0.022). Group R showed significant differences when the scores assigned to neutrophils at 7, 14, 30, 60 and 90 days were compared (p=0.017). In this group, it was observed that neutrophil infiltrate decreased over time.
DISCUSSION
The constant search for filling materials with adequate physical, chemical and biological properties has led the industry to develop new products that do not possess the limitations of materials already available in the market. The RealSeal System, which is composed of a resinous sealer and Resilon cones, was introduced into the market and, of late, has been used to fill canals18. This study evaluated the biocompatibility of the cones used in this filling system and compared them with gutta-percha cones and a control group.
The analysis of results revealed that the scores obtained for neutrophils in group R decreased over time. This demonstrated that the tissue response to Resilon cones was more intense in the initial period, which may be attributed to the presence of resinous components. In the GP and CG groups, scores obtained for neutrophils, eosinophils and abscesses were 1 and 2 at all the time intervals. This finding suggests that gutta-percha and polyethylene tubes are well tolerated by tissue.
The constant presence of lymphocyte and plasma cell infiltrates, macrophages and giant cells observed in the duration of the study characterizes a chronic inflammatory response to eliminate the cause of inflammation. The presence of fibrous tissue indicated that the organism was capable of maintaining materials isolated.
The results of this study are in agreement with findings of previous studies, which confirmed the biocompatibility of gutta-percha3,5,7,9.
Onay, Ozdemir and Ungor9 and Bodrumlu et al.10, using a method similar to that of the present study, found similar histological responses to implants of gutta-percha and Resilon cones. These authors found that the materials caused a moderate to severe inflammatory response after one week of contact with connective tissue. At 4 weeks, the reaction was classified as slight to moderate, and at 8 weeks, slight to absent. These findings are in agreement with our results, as the intensity of response in our study decreased with time.
The in vivo model used in our study is classified as a secondary trial to evaluate the biocompatibility of dental materials, and has been used in previous studies3,5-7,9,13-16. It simulated what happens with periapical tissue after the filling of root canals.
Secondary tissue tests are intended to identify products or product components with the potential to cause injury. Since they do not allow therapeutic responses in specific applications to be investigated, they are not by themselves sufficient to establish biocompatibility, but they do make it possible to assess tissue reactions in animal specimens, and is therefore an essential stage in the completion of assessments of the materials19-20.
The purpose of inserting polyethylene tubes was to standardize the area of contact of materials with subcutaneous tissue. Other studies used similar tubes and confirmed their inert nature19-20.
Material biocompatibility is linked to the amount and nature of a material's components. The greater the components' solubility, the more intense the inflammatory response may be. Cones used to fill root canals are expected to be stable and, consequently, inert. The results of our study confirm this hypothesis for the gutta-percha cones. Resilon cones caused more tissue irritation at the earlier time intervals, but were well tolerated after 30 days of contact with the connective tissue. Further studies should be conducted to describe the properties of Resilon cones.
CONCLUSION
The results of the present study demonstrate that Resilon cones are well tolerated by tissue and have acceptable biocompatibility, in the same way as guttapercha cones.
Collaborators
FS Grecca and GAX Acasigua were responsible for the bibliographical survey, project preparation, development of the experimental portion and the composition of the article. PMP Kopper developed the experimental portion, acquired the parts for the histological processing and took part in the composition of the article. RB SANTOS was responsible for the development of the experimental portion and the composition of the article. AC Fossati prepared the project, was responsible for the histological processing and the composition of the article. VC Carrard was responsible for the development of the experimental portion, the histological processing, the reading of the histological cuts and the composition of the article. JAP Figueiredo prepared the project and was responsible for the histological processing, the reading of the histological cuts and the composition of the article.
REFERENCES
1. Spangberg L. Endodontic treatment of teeth without apical periodontitis. In: Orstavik D, Pitt Ford TR (ed). Essential endodontology 1999. Cambridge, UK: Blackwell Science; 1999. p. 228. [ Links ]
2. Shipper G, Ørstavik D, Teixeira FB, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon). J Endod. 2004;30(5):342-7. doi: 10.1097/00004770-200405000-00009.
3. Leonardo MR, Utrilla LS, Rothier A, Leonardo RT, Consolaro A. Comparison of subcutaneous connective tissue responses among three different formulations of gutta-percha used in thermatic techniques. Int Endod J. 1990;23(4):211-7.
4. Nicholson RJ, Casanova F, Greenspan J, Stark MM. Comparison of tissue response between a synthetic gutta-percha and a natural gutta-percha endodontic filler. Oral Surg Oral Med Oral Pathol. 1975;39(5):802-5.
5. Sjogren U, Sundquivist G, Nair PN. Tissue reaction to guttapercha particles of various sizes when implanted subcutaneously in guinea pigs. Eur J Oral Sci. 1995;103(5):313-21. doi: DOI: 10.1111/j.1600-0722.1995.tb00032.x.
6. Tanzilli JP, Nevins AJ, Borden BG. The reaction of rat connective tissue to polyethylene tube implants filled with Hydron or guttapercha. Oral Surg Oral Med Oral Pathol. 1983;55(5):507-13.
7. Tavares T, Soares IJ, Silveira NL. Reaction of rat subcutaneous tissue to implants of gutta-percha for endodontic use. Endod Dent Traumatol. 1994;10(4):174-8. doi: 10.1111/j.1600- 9657.1994.tb00682.x.
8. Wolfson EM, Seltzer S. Reaction of rat connective tissue to some gutta-percha formulations. J Endod. 1975;1(12):395-402. doi: 10.1016/S0099-2399(75)80158-0.
9. Onay E, Ungor M, Ozdemir B. In vivo evaluation of the biocompatibility of a new resin-based obturation system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(3):e60-e66. doi: 10.1016/j.tripleo.2007.03.006.
10. Bodrumlu E, Muglali M, Sumer M, Guvenc T. The response of subcutaneous connective tissue to a new endodontic filling material. J Biomed Mater Res B. 2008;84(2):463-7. doi: 10.1002/ jbm.b.30892.
11. Holland R, Souza V, Nery MJ, Otoboni Filho JA, Bernabé PFE, Dezan Jr E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999;25(3):161-6.
12. Holland R, Souza V, Nery MJ, Faraco Jr IM, Bernabé PFE, Otoboni Filho JA, et al. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, portland cement or calcium hydroxide. Braz Dent J. 2001;12(1):3-8.
13. Kolokouris I, Economides N, Beltes P, Vlemmas I. In vivo comparison of the biocompatibility of two root canal sealers implanted into the subcutaneous connective tissue of rats. J Endod. 1998;24(2):82-5.
14. Molloy D, Goldman M, White RR, Kabani S. Comparative tissue tolerance of a new endodontic sealer. Oral Surg Oral Med Oral Pathol. 1992;73(4):490-3. doi: 10.1016/0030-4220(92)90332- K.
15. Zmener O, Guglielmotti MB, Cabrini RL. Biocompatibility of two calcium hydroxide-based endodontic sealers: a quantitative study in the subcutaneous connective tissue of the rat. J Endod. 1988;14(5):229-235.
16. Zmener O, Guglielmotti MB, Cabrini RL. Tissue response to an experimental calcium hydroxide-based endodontic sealer: a quantitative study in subcutaneous connective tissue of rat. Endod Dent Traumatol. 1990;6(6):66-72.
17. Figueiredo JAP, Pesce HF, Gioso MA, Figueiredo MAZ. The histological effects of four endodontic sealers implanted in the oral mucosa: submucous injection versus implant in polyethylene tubes. Int Endod J. 2001;34(5):377-85. doi: 10.1046/j.1365- 2591.2001.00407.x.
18. Garcia LF, Marques AA, Roselino LM, Pires de Souza FC, Consani S. Biocompatibility evaluation of Epiphany/Resilon root canal filling system in subcutaneous tissue of rats. J Endod. 2010;36(1):110-4. doi: 10.1016/j.joen.2009.09.012.
19. Holland R, Souza V, Nery MJ, Bernabé PFE, Otoboni Filho JA, Dezan Jr E, etal. Calcium salts deposition in rat connective tissue after the implantation of calcium hydroxide-containing sealers. J Endod. 2002;28(3):173-6.
20. Kaplan AE, Ormaechea MF, Picca M, Canzobre MC, Ubios AM. Rheological proprieties and biocompatibility of endodontic sealers. Int Endod J. 2003;36(8):527-32. doi: 10.1046/j.1365- 2591.2003.00683.x.
 Correspondence to:
Correspondence to:
FS GRECCA
e-mail: fabiana.grecca@ufrgs.br
Recebido em: 1/3/2011
Versão final reapresentada em: 26/4/2011
Aprovado em: 29/4/2011













