Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.4 Porto Alegre Out./Dez. 2012
ORIGINAL / ORIGINAL
Evaluation of integrity of procedure gloves used by dentistry students
Avaliação da integridade das luvas de procedimento usadas por alunos de Odontologia
Vinícius Motta PALERMO I; Ana Michele ZIMBALDI I; Flávia Martão FLÓRIO I; Luiz Carlos TEIXEIRA I; Almenara de Souza FONSECA-SILVA I
I Faculdade São Leopoldo Mandic, Curso de Odontologia. Campinas, SP, Brasil
ABSTRACT
Objective
To evaluate the integrity of procedure gloves, before and after clinical activities carried out by dentistry students.
Methods
480 gloves were analyzed, belonging to 4 different commercial brands, divided into groups of 60 gloves each: Group 1 - Sanro® (Fábrica de Artefatos de Látex São Roque S.A, São Roque, Brazil); Group 2 - Supermax® (Supermax Glove Manufacturing Sdn. Bhd. Selangor, Malaysia); Group 3 - Satari® (Siam Sempermd Corp., Ltd. 110 Kanjanavanit Rd., Hatyai, Thailand) and Group 4 - Embramac® (Hartalega Sdn. Bhd. Kuala Lumpur, Malaysia), comprising 240 unused (Control Group) and 240 used gloves. The gloves had been used by Integrated Clinic students for a period of up to 2 hours. The integrity, with regard to the strength of the glove material, was evaluated by visual inspection and a water insufflation test, having considered the following clinical conditions: presence of tearing, presence of small perforations characterized by trickling and presence of large perforations characterized by water discharges.
Results
Comparing the loss of integrity before and after use, one can observe that the values correspond to 5.4% and 27.1% respectively (p<0.0001), presenting significant differences between brands.
Conclusion
It may be concluded that the loss of integrity of gloves before and after clinical activities, exposes the dentistry students to the risk of contamination and this occurrence can vary depending on the brand of gloves used.
Indexing terms: Gloves protective. Infection. Students dental.
RESUMO
Objetivo
Avaliar a integridade de luvas de procedimento, sem uso e após o uso em atividades clínicas realizadas por alunos de Odontologia.
Métodos
Foram analisadas 480 luvas, de 4 marcas comerciais divididas em grupos de 60 luvas cada: Grupo 1 - Sanro® (Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brasil); Grupo 2 - Supermax® (Supermax Glove Manufacturing Sdn. Bhd. Selangor, Malaysia); Grupo 3 - Satari® (Siam Sempermd Corp., Ltd. 110 Kanjanavanit Rd., Hatyai, Thailand) e Grupo 4 - Embramac® (Hartalega Sdn. Bhd. Kuala Lumpur, Malaysia), sendo 240 luvas sem uso e 240 após o uso. As luvas foram usadas por alunos na disciplina de Clínica Integrada por um período de até 2 horas. A integridade, relacionada à resistência do material da luva, foi avaliada por inspeção visual e teste de insuflação de água, considerando as condições clínicas: presença de rasgos, presença de perfurações pequenas caracterizadas por gotejamento e de perfurações grandes caracterizadas por escoamento de água.
Resultados
Comparando a perda de integridade das luvas sem uso e após o uso, podemos observar que os valores correspondem a 5,4% e 27,1% respectivamente (p<0,0001), apresentando diferença significativa entre as marcas.
Conclusão
Conclui-se que a perda de integridade das luvas de procedimento, sem uso e após o uso em atividades clínicas, expõe os alunos de odontologia ao risco de contaminação e esta ocorrência pode variar em função da marcas de luvas usadas.
Termos de indexação: Luvas protetoras. Infecção. Estudantes de odontologia.
INTRODUCTION
Health professionals involved in the provision of dental services, including dental students, are potentially exposed to the risk of contamination by biological agents, characterized by exposure to bacteria, parasites, fungi, viruses, amongst others. Due to the proximity with patients' body tissues and fluids during clinical activities, both dental surgeons and their teams may come into contact with a large variety of pathogenic microorganisms that are capable of being transmitted1.
By virtue of the recognition that the biological risk could increase the possibility of damage to the health of these professionals, occupational safety has been gaining importance in the entire health sector, particularly after the publication in 2005 of the Brazilian Regulatory Standard NR322. The aim of this legislation was to establish the basic guidelines for implementing means of protecting the health and safety of health service workers. One of its recommendations is the use of personal protective equipment (PPE).
The use of personal protective equipment is an integral part of standard precautions, recognized infection control measures recommended by international health organizations such as the Center for Disease Control and Prevention3 and the World Health Organization (WHO)4.
Amongst these, gloves are recommended as physical barriers to avoid direct hand contact with organic matter, separating the tissues of patient and professional and providing simultaneous mutual protection. At the same time that they reduce contamination of the professional's hands by microorganisms belonging to the patient, they also reduce contamination of the patient by the microbiota on the hands of the professional5.
Procedure gloves are generally made from latex and are recommended for semi-critical clinical procedures where there is no invasion of the vascular system. They are manufactured as single-use materials, the use being recommended of a new pair for each patient, and being subsequently disposed of6-7.
Studies have demonstrated that gloves are not perfect barriers and that their capacity for protection is reduced when they are not completely intact. In addition to the lack of integrity related to manufacturing defects6, several studies have also witnessed a loss of barrier properties by latex gloves during routine consultations. After dental surgery, holes were found at a rate of 10.6%8 and 14.8%9. In the specialty of pediatric dentistry, the occurrence was found of 15% having holes in the gloves after use10, while in orthodontics these numbers were as high as 15.6%11. Otis & Cottone12 found damage varying between 38% and 44%, with only 5% of these being diagnosed using the naked eye.
Several reasons have been mentioned as responsible for the diminished integrity of latex gloves, such as prolonged use, humidity, intense manipulation of instruments and chemical products7,13. However, despite the limitations, latex gloves have demonstrated resistance against perforation during use, when compared to vinyl and nitrile gloves14-15, with the quality varying depending on the manufacturer16.
Assuming that, for the control of infection, the barrier property is effective if the gloves remain intact during consultation with the patient, it is proposed to evaluate the integrity of four makes of glove, before and after use, by the students at the São Leopoldo Mandic Faculty working on Integrated Clinic activities, by means of a questionnaire, visual inspection and using water insufflation.
METHODS
For this study, a total of 480 small-sized, ambidextrous, procedure gloves made of latex were evaluated, 240 of which were unused and 240 used by graduate dentistry students. The gloves, encompassing four different commercial brands, were divided into groups: Group 1 - Sanro Ambi® (Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brazil; Batch: 054061); Group 2 - SuperMax® (Supermax Glove Manufacturing Sdn. Bhd. Selangor, Malaysia; Batch: 51116822); Group 3 - Satari® (Siam Sempermd Corp., Ltd. 110 Kanjanavanit Rd., Hatyai, Thailand; Batch: 12200628140215) and Group 4 - Embramarc® (Hartalega Sdn. Bhd. Kuala Lumpur, Malaysia; Batch: 0609190654). A total of 60 unused gloves of each make were selected for analysis and another 60 were distributed for use as personal protective equipment for carrying out clinical procedures.
Twenty students enrolled in the graduation course at the São Leopoldo Mandic Faculty of Dentistry who were performing activities in the subject of Integrated Clinics, were invited to take part in the study. The project was approved by the Ethics in Research Committee and the work was only begun following agreement with and signing of the Free and Informed Consent form.
To be included in the study, the gloves would have to present adequate conditions of use as evaluated by visual inspection. Those with any defects, folds or viscosity were excluded. Each volunteer received a pair of procedure gloves at the beginning of the period of patient consultation at the Integrated Clinic, when the procedures were planned for calculus scraping, cavity preparations, restorations or endodontic treatment on patients, along with the glove usage instructions. Each pair of gloves was used by the volunteer for a period of up to 2 hours, and these were subsequently collected in individual plastic bags. The Study Questionnaire was then answered.
The gloves were analyzed in accordance with the following parameters: presence of tearing (Figure 1) evaluated by means of visual inspection; presence of large perforations characterizing discharges and the presence of small perforations characterized by trickling and identified during the water insufflation test, based on FDA standards17. The test consisted of filling the inside of the gloves with water at room temperature, with minimum handling and taking care to keep the outer surfaces dry. The readings were taken immediately and reconfirmed after 2 minutes of observation.
The data were initially analyzed by means of frequency distribution tables. After tabulation and analysis of the data, the Fisher exact test was employed. All testing was carried out using the SAS statistical software application. Statistically significant associations were considered to be those where the values of p (probability of a type 1 error) were less than or equal to 5% (0.05), in 2-tailed distribution.
RESULTS
The sample comprised 480 gloves, half of which were unused while the other half were used in clinical activities, and were divided into 4 groups of 60 gloves each (Group 1 - Sanro®, Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brazil; Group 2 - Supermax®, Supermax Glove Manufacturing Sdn. Bhd. Selangor, Malaysia; Group 3 - Satari®, Siam Sempermd Corp., Ltd. 110 Kanjanavanit Rd., Hatyai, Thailand and Group 4 - Embramac® (Hartalega Sdn. Bhd. Kuala Lumpur, Malaysia). The occurrence of loss of integrity related to the glove material was evaluated using three parameters: occurrence of tearing, large perforations or small perforations.
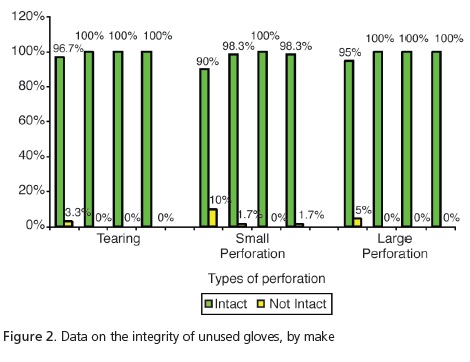
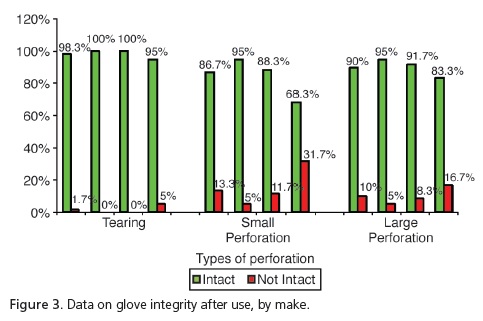
The analysis of the unused gloves (Figure 2) demonstrated that, despite group G1 having presented 3.3% with tears and 5% more large perforations in relation to the other groups, these differences were not significant (p=0.2469 and p=0.0617, respectively). However, when considering the presence of small perforations, significant differences were observed (p=0.0145), with group G1 presenting the highest number of non-intact gloves (10%) in relation to the group G2: 17% and G4: 1.7%. As for the unused gloves, group G3 had no perforations at all. The analysis of the gloves after use (Figure 3) demonstrated that, despite group G4 presenting tearing of 5% in comparison with G1 at1.7%, there was no significant difference (p=0.1972). As regards the presence of large perforations, G1=10%; G2=5%; G3=8.3% and G4=16.7%, there were also no significant differences (p=0.2402). However, when considering the presence of small perforations, significant differences (p<0.001) were observed, with higher percentages for group G4=31.7% in relation to the other groups (G1=13.3%; G2=5%; G3=11.7%).
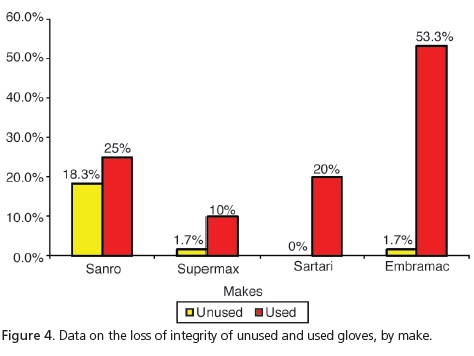
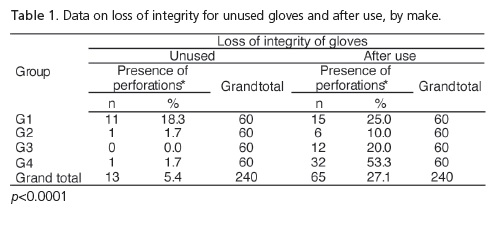
Taking into consideration the 3 types of perforation simultaneously, which characterize loss of integrity, Table 1 and Figure 4 show that the use of gloves stimulates the occurrence of perforations and there is a significant difference when considering the loss of integrity by make and by use.
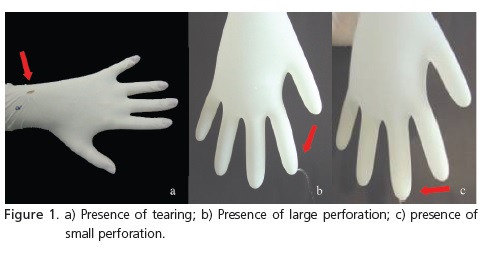
DISCUSSION
Every dental surgeon, in the exercise of his/ her profession, comes into contact with patients' body tissue and fluids such as saliva and blood, as well as microorganisms arising from these fluids, making them vulnerable to diseases borne by viruses, bacteria or fungi18. In order to reduce occupational risks, the use of personal protective equipment is recommended, defined as devices for personal use used by the worker and intended to prevent risks that could threaten health and safety19. Amongst these, gloves are recommended as physical barriers to avoid the professional's hands coming into direct contact with organic matter5. Studies have shown, however, that the barrier property is diminished when gloves lose their integrity8-13.
Guandalini20 stated that in one box of ambidextrous, latex procedure gloves, up to 12% of these gloves may have manufacturing defects. In the present study, one of the steps included the evaluation of 240 unused procedure gloves based on the following parameters: presence of tearing, small perforations or large perforations. The results corroborate those of Guandalini20, since 5.4% of the sample was found to have perforations. Small perforations occurred with the make Sanro (Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brazil) and 10% and 1.7% respectively with Supermax (Supermax Glove Manufacturing Sdn. Bhd. Selangor, Malaysia) and Embramac (Hartalega Sdn. Bhd. Kuala Lumpur, Malaysia). As for tearing and large perforations with Sanro (Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brazil) we obtained 3.3% and 5%, respectively. Therefore, taking into account the total number of perforations leading to loss of integrity, Sanro (Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brazil) showed significantly higher results (18.3%), when compared with other makes. Accordingly, a reduction in the barrier property was found with a consequent increase in user exposure to biological risks.
With the aim of improving the quality control of gloves, the National Health Surveillance Agency (ANVISA) published resolution RDC 5, which came into force on January 1, 2009. This legislation establishes a specific regulation for surgical and procedure gloves defining the minimum criteria for the identification and quality of gloves manufactured in Brazil and those imported commercially into the country, thereby ensuring the sale of safer, more effective personal protection equipment21.
Out of a total of 240 procedure gloves analyzed, after use in the Integrated Clinic course subject, it was shown that there was a loss of integrity in 27.1% of the sample. Statistically speaking, this value is higher when compared to data for unused gloves (5.4%). It was noted that ruptures occurred with all commercial makes examined, whether when putting them on or taking them off or on account of the presence of small or large perforations. We can see that with small perforations, the make Embramac (Hartalega Sdn. Bhd. Kuala Lumpur, Malaysia) had 31.7% followed by 13.3% Sanro (Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brazil), 11.7% Satari (Siam Sempermd Corp., Ltd. 110 Kanjanavanit Rd., Hatyai, Thailand) and 5% Supermax (Supermax Glove Manufacturing Sdn. Bhd. Selangor, Malaysia) and with regard to large perforations, we found Embramac (Hartalega Sdn. Bhd. Kuala Lumpur, Malaysia) with 16.7%, Sanro (Fabrica de Artefatos de Látex São Roque S.A, São Roque, Brazil) with 10%, Satari (Siam Sempermd Corp., Ltd. 110 Kanjanavanit Rd., Hatyai, Thailand) with 8.3% and Supermax (Supermax Glove Manufacturing Sdn. Bhd. Selangor, Malaysia) with 5%. These results are consistent with Cury22, who evaluated 203 pairs of surgical gloves in procedures of general surgery and found that 23.2% of gloves had significant perforations. These results were also in agreement with those of Pinheiro et al.10 and Leal et al.11, who found perforations in 15%, on average, and with the study performed by Reis et al.23, who demonstrated a rate varying between 15.5% and 37%.
One of the probable causes for the increase in loss of integrity values after clinical procedures relates to the increase in permeability caused by chemical products used in dentistry, such as solvents, acids, whiteners24-25. Other factors involved in the fragility of gloves which are usually indicated include bad quality of material, use of inadequate numbering and lack of accuracy of prior quality control tests26.
Tarantola27 explains the presence of perforations as being the result of the manufacturing process, since in the method known as immersion molding, via which latex gloves are manufactured, the filling of a hand-shaped mold takes place using a thin, liquid layer of latex. Due to the fineness and high fluidity of latex, pinholes could appear on the surface of the material, as it could also during the polymerization stage. Thus, during clinical procedures where humidity is present and mechanical forces are exerted, preexisting perforations could expand significantly.
In the present study, the gloves were used for the purposes of carrying out the scraping of calculus, cavity preparation, restorations or endodontic treatments, routine dental procedures, normally characterized by the intensive use of the hands, generating friction and stress in the fingers. In this way, a higher incidence of afteruse perforations might suggest that some materials or processes used in the manufacture of gloves could result in products that are not strong enough to be used as personal protective equipment for those dental procedures which demand intense manipulation of instruments.
Given the potential risk of hand contamination, it is recommended that cuts and abrasions be covered with waterproof dressings before putting on the gloves and also that they be washed after removal to increase occupational safety3,7. Knowledge of biosafety together with the adoption of preventive measures can help to reduce the risk of infection in dentistry.
CONCLUSION
Given the limitations of the present study, it may be concluded that the loss of integrity of unused gloves and gloves after use in clinical activities, exposes dental students to the risk of contamination and this can vary according to the make of the gloves.
Collaborators
VM PALERMO, AM ZIMBALDI, FM FLÓRIO, LC TEIXEIRA and ASouza FONSECA-SILVA participated in the conception, analysis, data interpretation, and final review of the study.
REFERENCES
1. Estrela C, Estela CRA. Controle de infecção em odontologia. São Paulo: Artes Médicas; 2003. [ Links ]
2. Brasil. Ministério do Trabalho e Emprego. Portaria n. 485, de 11 de novembro de 2005. Aprova a Norma Regulamentadora - NR 32, relativa à Segurança e Saúde no Trabalho em Serviços de Saúde [texto na Internet]. Diário Oficial da República Federativa do Brasil, Brasília (DF); 16 nov. 2005; seção 1. Disponível em: <http://www.anvisa.gov.br/servicosaude/avalia/saude_do_ trabalhador_portaria_485_aprova_NR32.pdf>.
3. Center for Disease Control and Prevention (CDC). Guidelines for infection control in dental health-care settings - 2003. MMWR Recomm Rep. 2003;52(RR17):1-67.
4. World Health Organization (WHO). Aide-Memoire - Standard precautions in health care. Geneva, 2007 [cited 2009 5 Apr]. Available at: <http://www.who.int/csr/resources/publications/ EPR_AM2_E 7.pdf>.
5. Fonseca-Silva AF, Risso M, Ribeiro MC. Biossegurança em ambientes odontológicos. São Paulo: Pancast; 2004.
6. Guandalini SL, Melo NSFO, Santos ECP. Biossegurança em odontologia. Curitiba: Odontex; 1998.
7. Fonseca-Silva AF, Risso M, Ribeiro MC. Biossegurança em odontologia e ambientes de saúde. 2 ed. São Paulo: Ícone; 2009.
8. Teixeira AR, Fernandes RA, Serratine ACP. Perfurações em luvas de látex utilizadas em cirurgias odontológicas. Odontol Clín Científ. 2008;7(2):145-50.
9. Serratine ACP, Pacheco E, Miero M. Avaliação da integridade de luvas cirúrgicas após a utilização em cirurgias odontológicas. Arq Catar Med. 2007;36(1):85-9.
10. Pinheiro JT, Aguiar CM, Dantas MAT. Avaliação da integridade das luvas de procedimento utilizadas na clínica odontológica. Rev ABO Nac. 2005;13(5):287-92.
11. Leal MHC, Pinheiro JT, Aguiar CM, Leão EC. Avaliação da integridade das luvas de procedimento utilizadas na clínica ortodôntica. RGO - Rev Gaúcha Odontol. 2004;52(4):251-5.
12. Otis LL, Cottone LA. Prevalence of perforations in disposable latex gloves during routine dental treatment. J Am Dent Assoc. 1989;118(3):321-4.
13. Richards JM, Sydiskis RJ, Davidson M, Josell SD, Lavine DS. Permeability of latex gloves after contact with dental materials. Am J Orthod Dentofacial Orthop. 1993;104(3):224-9.
14. Rego A, Roley L. In-use barrier integrity of gloves: latex and nitrile superior to vinyl. Am J Infect Control. 1999;27(5):405-10. doi: 10.1016/S0196-6553(99)70006-4.
15. Korniewicz DM, Garzon L, Seltzer J, Feinleib, M. Failure rates in non-latex surgical gloves. Am J Infect Control. 2004;32(5):268- 73. doi: 10.1016/j.ajic.2003.12.005.
16. Pitten FA, Herdemann G, Kramer A. The integrity of latex gloves in clinical dental practice. Infection. 2000;28(6):388-92. doi: 10.1007/s150100070011.
17. Food and Drug Administration (FDA). Code of Federal Regulations CFR 21 Part 800. Medical devices; patient examination and surgeon's gloves; test procedures and acceptance criteria. EUA: FDA; 2006.
18. Chinellato LEM, Scheidt WA. Estudo e avaliação dos meios de biossegurança para o cirurgião-dentista e auxiliares contra doenças infecto-contagiosas no consultório odontológico. Rev Fac Odontol Bauru. 2003;1(1/4):60-6.
19. Brasil. Ministério do Trabalho e Emprego. Norma Regulamentadora n. 6 - Equipamento de proteção individual. Brasília: Ministério do Trabalho e Emprego; 2002 [citado 2009 5 Abr]. Disponível em: <http://portal.mte.gov.br/data/ files/8A7C816A33EF45990134335D0C415AD6/NR-06%20 (atualizada)%202011.pdf>.
20. Guandalini SL. Biossegurança. JBC J Bras Odont Clin. 1997;1(1):9-11.
21. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. RDC n. 5, de 15 de fevereiro de 2008. Estabelece os requisitos mínimos de identidade e qualidade para as luvas cirúrgicas e luvas de procedimentos não-cirúrgicos de borracha natural, borracha sintética ou mistura de borrachas natural e sintética, sob regime de vigilância sanitária. Diário Oficial da República Federativa do Brasil, Brasília (DF); 18 fev 2008 [citado 2009 Abr 5]. Disponível em: <http://www.anvisa.gov.br/divulga/ noticias/2008/190208_RDC_5.pdf>.
22. Cury AF. Perfuração da luva cirúrgica: freqüência e percepção do acidente. Rev Bras Ginecol Obstet. 1999;21(10):593-6. doi: 10.1590/S0100-72031999001000005.
23. Reis LDO, Lombarda OA, Reis ADO. Luvas e sua eficiência em proteger a equipe cirúrgica de infecções. Rev Col Bras Cir. 1990;17(1):19-22.
24. Nakamura M, Oshima H, Hashimoto Y. Monomer permeability of disposable dental gloves. J Prosthet Dent. 2003;90(1):81-5. doi: /10.1016/S0022-3913(03)00178-1.
25. Tinsley D, Chadwick RG. The permeability of dental gloves following exposure to certain dental materials. J Dent. 1997; 25(1):65-70. doi: 10.1016/0300-5712(95)00124-7.
26. Gunasekera PC, Fernando RJ, Silva KK. Glove failure: an occupational hazard of surgeons in a developing country. J R Coll Surg Edinb. 1997;42(2):95-7.
27. Tarantola A. Of viruses, gloves, and crêpes. Am J Infect Control. 2007;35(4):284. doi: 10.1016/j.ajic.2006.08.004.
 Correspondence to:
Correspondence to:
VM PALERMO
e-mail: vmpalermo@yahoo.com.br
Received on: 11/12/2008
Final version resubmitted on: 9/4/2009
Approved on: 22/6/2009













