Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.4 Porto Alegre Out./Dez. 2012
ORIGINAL / ORIGINAL
Evaluation of antimicrobial contamination and resistance to Staphylococcus aureus collected from radiographic materials used in dentistry
Contaminação e resistência antimicrobiala de staphylococcus aureus colhidos em materiais de processamento radiográfico em Odontologia
Rachel Medeiros dos SANTOS I; Fábio Luiz Medeiros dos SANTOS II; Juliana Cama RAMACCIATO I; José Luiz Cintra JUNQUEIRA I
I Faculdade São Leopoldo Mandic, Curso de Odontologia, Programa de Pós-Graduação em Radiologia. Campinas, SP, Brasil
II Universidade UNIGRANRIO, Curso de Odontologia. Rio de Janeiro, RJ, Brasil
ABSTRACT
Objective
The aim of this study was to quantify Staphylococcus aureus isolated from materials used in radiographic processing, as well as to determine their sensitivity to antimicrobial agents commonly used in dentistry.
Methods
Samples were collected at the endodontics clinic of São Leopoldo Mandic Dental School, Campinas, São Paulo, Brazil and then inoculated in brain heart-infusion (BHI) agar and mannitol salt agar. After incubation at 37°C for 24 hours, colony-forming units (cfu/ml) were counted. Commercial paper disks containing widely prescribed antimicrobial agents were used to perform the antibiotic susceptibility tests.
Results
The highest bacterial contamination was observed in the lids of the portable dark rooms. The highest bacterial resistance rates were observed for erythromycin (60%) and the beta-lactam group: penicillin G (25%); ampicillin (18%); and amoxicillin (28%).
Conclusion
In conclusion, the present study highlights the need to establish strategies to prevent bacterial cross-contamination during radiographic procedures in dental settings.
Indexing terms: Contamination. Radiology. Staphylococcus aureus.
RESUMO
Objetivo
Verificar a contaminação e a resistência antimicrobiala de Staphylococcus aureus isolados das tampas das câmaras escuras portáteis e das soluções reveladora e fixadora.
Métodos
As amostras foram coletadas ao final do dia de trabalho, na clínica de Endodontia da Faculdade São Leopoldo Mandic - Unidade Campinas, São Paulo. As amostras foram inoculadas em meios sólidos BHI (brain heart infusion) e salt manitol e posteriormente incubadas a 37°C por 24 horas para leitura do número total de unidades formadoras de colônias por mililitro (ufc/ml). Após certificar-se de que a colônias crescidas eram de Staphylococcus aureus, foi feito o repique de 1 ou 2 colônias em BHI líquido, para preparo um inoculo de 108 ufc/ml de Staphylococcus aureus. Posteriormente este inóculo foi semeado em placas contendo müeller-hinton ágar para a realização do Teste de Sensibilidade Antimicrobiala.
Resultados
Foi observada contaminação por Staphylococcus aureus nas câmaras escuras portáteis e nas soluções, sendo maior prevalência nas amostras obtidas nas câmaras escuras portáteis (82%). Maiores porcentagens de resistência do micro-organismo foram observadas para a eritromicina (60%) e os antibióticos do grupo das penicilinas: penicilina G (25%), ampicilina (18%) e amoxicilina (28%). O microorganismo demonstrou pequena porcentagem de resistência para demais antibióticos testados (cloranfenicol, claritromicina, oxacilina, clindamicina, tetraciclina, vancomicina e cefadroxil).
Conclusão
Estes resultados demonstram a necessidade de medidas que evitem a contaminação-cruzada durante os procedimentos radiológicos em odontologia.
Termos de indexação: Contaminação. Radiologia. Staphylococcus aureus.
INTRODUCTION
In the field of Dental Radiology, during clinical practice, contact with drops of saliva, splashes of blood, invisible pieces of body tissue and secretions occur all the time. Accordingly, the propagation of infectious diseases is made possible via cross-contamination1.
Blood and saliva can transport viruses and pathogenic bacteria which could cause anything from the common cold to other more serious diseases such as labial herpes, hepatitis B and C, pneumonia, tuberculosis and, more rarely, acquired immunodeficiency syndrome (AIDS). By neglecting controls over cross-infection, the risk of infections in patients and professionals grows larger.
Those microorganisms that are potential indicators of contamination in a dental environment include Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mutans, Streptococcus mitis, Streptococcus salivarius, Corynebacterium diphtheriae, Bacteroides fragilis and Peptoestreptococus.
Dental Radiology may be regarded as a specialty that has the potential for the occurrence of crosscontamination. During radiographic processing, after placing the radiographic film in the developing solution, passing through water and in a fixing solution for manual processors, it was found that the films remained contaminated, even 48 hours after radiographic exposure2.
On analyzing the control of cross-infection in Dental Radiology, it was found that the majority of radiology clinics in Faculties of Dentistry do not perform prior disinfection of the x-ray apparatus and other locations where radiographic processing takes place.
The need to use aseptic techniques is an attempt to reduce and/or eliminate the risk of cross-contamination during the taking and processing of x-rays. Included in the description of the biosafety procedures in Dental Radiology are care with prior disinfection of the location where the patient is being treated, the use of PVC film on the x-ray equipment and radiographic films, and the use of Personal Protective Equipment (PPE) by the professional, amongst other care3.
Amongst the various types of microorganisms found in the oral cavity, S. aureus are considered to be one of the most versatile and dangerous human pathogens.
In Dentistry, few studies have evaluated the pattern of antimicrobial resistance to isolated strains of S. aureus in the clinical environment and in dental procedures. Studies such as those of Bernardo et al.4, who demonstrated strains of this microorganism that were resistant to ampicillin in the air of a dental clinic, and that of Motta et al.5 who isolated strains with a high level of resistance in locations such as the power button for the chair, triplex syringe and the cone of x-ray apparatus; they demonstrate the importance of studying this microorganism in Dentistry.
In Radiology, no studies were found that evaluated the level of resistance of S. aureus to the antibiotics most frequently used in Dentistry. From this emerged the interest in evaluating the contamination of materials used in the processing of intraoral x-rays and the degree of antimicrobial resistance of these isolated strains.
Cross-contamination in dental procedures
Cross-contamination may be defined as the transmission of infectious agents between patients and teams, within a clinical environment. Transmission may occur from person to person or via contaminated objects. In Dentistry, the source of infection could include patients who suffer from infectious diseases, those who are in the prodromal period of certain infections and healthy carriers of pathogenic strains. In the prodromal period, although the patient may appear healthy, his saliva and blood could be contaminated. In the case of asymptomatic carriers, the individual does not present with a history of infection, however he could have infectious microorganisms in the saliva or the blood.
In recent decades, confining contamination in dental surgeries has been a major challenge. For centuries, dental professionals have carried out their work oblivious to the risks of contamination inherent to their practice, until such time as it started to be understood that infections could be transmitted inside the dental surgery.
Dentists are exposed to a variety of pathogenic microorganisms arising out of dental treatment. Exposure may occur through direct contact with blood or saliva; or indirectly through the instruments, equipment and surfaces in the contaminated working environment.
The main route of dissemination of infection in dental surgeries is through direct contact with the body fluids of the sick patient, contact with surfaces or with instruments contaminated by the patient and contact with the patient's infectious particles which contaminate the air.
In the dental clinic, the biggest source of infection is the patient's mouth. In the oral cavity, more than 500 species of microorganism6 have been identified belonging to 30 different genera, constituting a diversified microbiota which usually survive in equilibrium. The dorsum of the tongue, the periodontium, the gingival groove and the dental plate are sites which are suited to the proliferation and maintenance of microbiota.
The oral cavity is filled with fluids containing viruses and bacteria which contaminate the saliva. The upper or subgingival dental plate is the biggest source of microorganisms. Moreover, through oronasal contact, there could be a transfer of pathogenic microorganisms that were acquired through the airways and which will be present in the saliva and oral fluid7.
This highly colonized environment, allied with the work of the dental surgeons with their low and high speed rotating instruments, frequently connected to systems comprising jets of water or air, result in the contamination of the consulting rooms, due to the production of aerosols with contaminated particles7.
During routine work, saliva may not be visible and act as a source of contamination. Improper use of procedures for cleaning and disinfecting surfaces between the appointments of one patient and the next, the lack of care in the use of Personal Protective Equipment and in the handling of dental materials could favor crosscontamination. In dentistry, the potential for crosscontamination is extremely high, particularly when intraoral x-rays are exposed and processed8.
Rahmatulla et al.9 reported that the sources of serious infection for the patient and for the team working in the dental surgery, have been blood, saliva and water used in the dental equipment. In radiological equipment, the places that are touched by the hand are potential areas for infection. These authors evaluated the contamination of radiological equipment and ascertained that practically all the areas of the equipment that were touched by the hands of the professionals and which did not undergo any kind of disinfection, were contaminated.
White & Glaze10 ascertained that oral microorganisms were transferred from patient to the radiographic equipment and from this to the next patient. These authors evaluated microbiological contamination of patients after radiographic exams. They found that it was possible for there to be transfers of Streptococcus pyogenes, S. aureus and Diplococcus pneumoniae to patients in the order of 30%, the main vectors being the contaminated hands of the technician and the radiological equipment. It was noted that these micro-organisms could survive for at least 48 hours on the surfaces of the x-ray apparatus.
The contamination of the environment of a radiology room and the surface of an automatic radiographic processor was found, before and after clinical activity. Greater levels of contamination were observed during clinical activities, the places of greatest contamination being the air and the door into the room, in comparison with the contamination of developing solutions, fixer and water. The authors did not aim to identify the microorganisms present, but rather to alert to the risk of contamination and the need for preventive measures2.
Lawson et al.11 found that potentially pathogenic bacteria like Escherichia coli, Enterococcus faecalis and S. aureus can survive for long periods in radiographic image receptors, alerting to the need for measures to control and prevent nosocomial infections.
According to the authors Hardman et al.12, contamination can also cause effects that interfere with the image quality of radiographic film, such as alteration in the density and contrast of radiographic images.
Other sources of contamination in dental clinics come from high-speed rotating instruments that contribute significantly to the contamination of consulting rooms via aerosols, such as to the dentist's hands, and in this way contamination is propagated to the faucets in the sinks and to the power buttons of the dentist's chair.
Bacterial resistance - Staphylococcus aureus
Aerosols generated by dental procedures are predominantly Streptococcus and Staphylococcus spp. S. aureus are common skin microorganisms with the characteristic of an opportunistic pathogen and with the potential to survive on surfaces for a long period of time13. These microorganisms have demonstrated a worrying increase in resistance to antibiotics such as methicillin and vancomycin.
Streptococcus is the principal cause of bacterial endocarditis which resides in the skin and the respiratory tract. Neisseria are oral species more commonly found in the mucus and saliva, however they are not usually pathogenic.
Infection is the deposit of a microorganism in the tissue. The number of microorganisms needed to cause an infection is called an "infectious dose". The infectious dose depends on factors such as the virulence of the Fmicroorganism and the health of the host. For example, large quantities of S. aureus can be applied to skin which is intact without causing a clinical infection; however, in the presence of a suture, as many as 100 cells could be enough to trigger a clinical infection.
There are numerous studies in the literature demonstrating the presence of pathogenic microorganisms like methicillin resistant S. aureus (MRSA) and Vancomycin Resistant Enterococci (VRE), responsible for cases of morbidity and death in hospitalized patients13-17.
Methicillin resistant S. aureus (MRSA) demonstrated significant rates of mortality in elderly patients and the immunocompromised, but is less prevalent and less fatal in younger patients. They are not often found in healthy hosts; however when isolated, they can be an indicator of a patient's clinical debility18.
Infections caused by S. aureus are often preceded by the colonization of the body, in particular the airways19. Strains of methicillin resistant and oxacillin resistant S. aureus are significant biological contaminants in clinical environments, mainly due to ever-increasing bacterial resistance. The literature has reported the isolation of resistant strains including vancomycin in hospitals.
Kurita et al.20 demonstrated that methicillin resistant S. aureus were isolated from the surface of a triplex syringe and dental chair in the Oral Surgery at the University of Shinshu. Eight of the 140 patients were contaminated by this microorganism and it was found that those isolated from the patients were from the same strain as those isolated from the clinic, demonstrating that the environment where dental treatment takes place can be considered a risk for cross-contamination, including potentially pathogenic microorganisms.
Biosafety and cross-contamination in dental radiology
At the present time, in spite of the enormous scientific and technological progress, cross-contamination still represents a risk in dental practice. In environments where professionals and patients meet, involved in clinical work, as in Dental Faculties, the risk of contamination is high, and measures for the control of asepsia in these locations are extremely necessary.
Dental surgeons, assistants and patients come directly into contact with a large quantity of pathogenic and non-pathogenic microorganisms, arising from oral microbiota and patient saliva or from the environment of the dental surgery, which may cause and transmit disease, ranging from common colds to more serious diseases such as pneumonia, tuberculosis, acquired immunodeficiency syndrome (AIDS), hepatitis B, hepatitis C, labial herpes, Human Papillomavirus (HPV), amongst others.
In dentistry, potentially pathogenic microorganisms can be transmitted from the patient's mouth or contaminated materials to the dentist's hands and then on to other materials and equipment used in the dental treatment20.
Saliva and nasopharyngeal secretion may contain pathogenic microorganisms such as the influenza virus, herpes virus, pathogenic Streptococcus and Staphylococus7. With the concern over controlling these infections, it is necessary to employ biosafety measures.
The area of Dental Radiology is not normally associated with needles, cutting instruments and aerosols. However, contact with saliva, blood splashes, tissue or secretions occur with some frequency. The potential for cross-infection between the professional team and the patients is considerable when patients are exposed to intraoral radiography due to contact with contaminated saliva. Other forms of contamination occur via the contaminated environment of processing rooms and during the process of developing radiographic films21.
There are two ways of carrying out asepsia of the surfaces in the dental surgery environment: preventing contamination with the protection of the surfaces and using pre-disinfection and disinfection measures after use.
Measures of sterilization, disinfection, use of protective barriers and aseptic handling of radiological equipment and materials are the means advocated for the prevention of microorganism transmission in radiological procedures.
The Brazilian Ministry of Health counts radiographic film amongst those surfaces that are capable of being contaminated and at the same time difficult to disinfect. Accordingly, a certain diligence is required to avoid contamination of radiographic film, including: wrapping it in plastic or using disinfectant solutions on the protective cover of the radiographic film before the process of development. These measures are not regarded as harmful to the quality of the images obtained22.
The American Dental Association (ADA) and the American Academy of Oral and Maxillofacial Radiology (AAOMR) recommend that, to prevent the contamination of radiographic film, the glove which is used to remove the film's plastic protection should not be the same as the one used during development of the film.
Coogan et al.23 evaluated the effectiveness of disinfectant substances like sodium dichloroisocyanurate and alcohol-phenol-iodide on radiographic films contaminated with Candida albicans, Streptococcus mutans and Lactobacilli. They found that the disinfectant solutions eliminated around 99.8% of the microorganisms present.
In radiological procedures studies were found, such as those described previously, which mainly evaluated the contamination and the institution of possible biosafety measures in Radiology. However, little is known about the level of resistance of the microorganisms isolated during this procedure.
Identification of Staphylococcus aureus
The main species of staphylococci found in human beings are S. aureus and S. epidermidis. The latter is found primarily residing in the skin and is not considered to be pathogenic. S. aureus, on the other hand, is a potential pathogenic and may be present in the nasopharynx of up to 40% of individuals. It is probably from this location that the species manages to reach the oral cavity, where it may occasionally be detected. This species does not play a significant role in intraoral infections, but they can cause serious infection associated with accidental surgical wounds.
To correctly identify the bacteria, a description of some of their more important characteristics is required. As regards format, the bacteria possess two fundamental morphological types: spheres (cocci) and rods (bacilli). The cocci take on different denominations according to their grouping: grouped spheres in the shape of a bunch of grapes constitute a staphylococcus; it forms chains, streptococcus; and when they come together in groups of two, diplococcus. The name bacilli is given to rod-shaped bacteria; more exclusively, however, they call bacilli those rods whose extremities are cut at right angles.
Using Gram's staining technique, which is described in more detail below, the microscopic characteristics of the staphylococci are as follows: they are colored purple manifesting themselves in the form of cocci in pairs, of bunches of grapes or grouped. Another way to identify streptococci and staphylococci is based on the morphology they present in culture media. The evaluation of the macroscopic characteristics of colonies is usually performed by way of a visual inspection of growth on the surface of petri dishes containing culture media. The colonies of staphylococci are bigger, convex in shape, with coloring ranging from porcelain white to yellow and may or may not present with hemolysis. Colonies of streptococci tend to be smaller, punctiform and with full or partial, hemolytic halos. The distinction between streptococci and staphylococci is arrived at more surely using a catalase test. They can cause some confusion when being compared using Gram coloration as they are eliminated, by adding a few drops of H2O2 at 3% to the colony. If O2 bubbles are detected then the catalase is present and the microorganism must be staphylococcus; the absence of bubbles indicates that the microorganism must belong to the genus streptococci.
The majority of tests used to evaluate the biochemical or metabolic activity of bacteria, through which a final identification of the species may be performed, are carried out by means of subcultures of the primary isolation in a series of distinct media, the results of which may be interpreted after at least one day of additional incubation. To identify bacteria, other tests may be used such as: coagulase test, hippurate hydrolysis, hydrolysis of arginine, Lactose fermentation, amongst others, it being necessary to use standard tables to compare results in order to determine the species of the microorganism being studied24.
S. aureus has the ability to ferment the mannitol in a medium containing 7.5% sodium chloride, called mannitol salt agar or Chapman's medium. The pH indicator is phenol red, which shows a positive reaction when the medium around the colonies turns yellow and negative when it remains reddish.
Gram staining
Gram staining is the bacterioscopic method most frequently employed in bacteriology at the present time, aiming to classify microorganisms based on their characteristics of tincture, size, form and cell arrangement25.
The method is based on the fact that, when bacteria are colored using gentian violet (or by dyes such as crystal violet or methyl violet) and then treated with iodine (Lugol's solution), a compound is formed with a dark coloring between the iodine and the dye (iodinepararosaniline), which is heavily retained by gram-positive bacteria and cannot be easily removed by subsequent treatment using alcohol. Gram-negative bacteria are easily discolored with alcohol. Safranin or fuchsine then colors the structures that were discolored by the alcohol. In this way, the gram-negative bacteria will appear red, while the gram-positive bacteria will appear purple, as they keep the violet color26.
The explanation for the coloring mechanism relates to the difference in composition of the cell wall in Gram-positive and Gram-negative bacteria. The cell wall is an external reinforcement of the cytoplasmic membrane of the bacteria, whose main function is to maintain the morphology and hypertonicity of the medium, acting as an osmotic barrier and affording the bacteria the property of maintaining elements inside them essential to their survival.
Its constitution varies from species to species and between Gram-positive and Gram-negative, however the presence of a mucopeptide (peptidoglycan) formed by amino sugars, is common to all. In Gram-positive microorganisms, the mucopeptide corresponds to between 40% and 90% of the composition of the cell wall, while in Gram-negative microorganisms it is between 4% and 10%. Bacteria that have a cell wall composed of murein (peptidoglycan - peptide of n-acetylmuramic acid), during the process of discoloration using ethyl alcohol, retain the dye. On the other hand, bacteria with cell walls primarily composed of fatty acids (lipopolysaccharides and lipoproteins) lose the iodine-pararosaniline complex, taking on the color of the background dye. Gram stains should be observed with the aid of an objective lens of small magnification (10x) to be able to see the global coloration, thickness and for the evaluation of somatic cells; the microorganisms should be observed under an oil immersion objective lens (100x)25.
Antibiotic Susceptibility Test (AST)
Susceptibility tests are recommended for any organism responsible for an infectious process that requires antimicrobial therapy, when it is impossible to predict the sensitivity of this organism, even after identification. Susceptibility tests are recommended most frequently when it is thought that the causal organism belongs to a species capable of presenting resistance to the antimicrobial agents normally used.
Penicillins, cephalosporins, macrolides and tetracyclines are the main antibiotics used in dentistry, clindamycin being an alternative to penicillin V in the treatment of dental infections caused by anaerobic microorganisms27. Penicillins are the antibiotics of first choice for the treatment of mild to moderately severe dental infections. Some cephalosporins are recommended for use in hospitals, such as cefadroxil.
In the past, bacterial sensibility tests were not standardized. The first studies performed with the aim of standardizing the antibiogram methodology were developed by the Food and Drug Administration (FDA) in 1972 and the World Health Organization (WHO) in 1977. Later on, a consensual standardization was adopted between these entities and the National Committee for Clinical Laboratory Standards (NCCLS), which in January 2005 changed its name to the Clinical Laboratory and Standards Institute (CLSI)28.
For the susceptibility tests in a solid medium, agar is used as a gelling agent for solid media to which are added selected nutrients, depending on the nutritional requirements of the bacterial species to be studied. It is a complex of natural substances derived from marine algae, which contain two types of polysaccharides (agarose and agaropectin) and metal cations, as well as other elements. It is a gel primarily composed of water, thereby allowing the diffusion of substances from areas of high concentration to low concentration24.
Various laboratory methods can be used to measure the in vitro sensitivity of the bacteria to the antimicrobial agents. In many microbiology laboratories, the method known as disk-diffusion or agar-diffusion is used, where the antibiotic (impregnated on paper discs) is applied to the petri dish containing the scattered medium. The antimicrobial spreads in centrifugal fashion causing a gradient with a lower concentration as it moves way from the center, where the disk is located, until it is no longer sufficient to inhibit growth. This gradient is affected by the drug's ability to spread through the agar and by the rate of bacterial growth, and at the limit of the inhibition halo that has formed, a critical population of bacteria can be found. Due to the nature of the diffusion test, both the concentration of the drug on the disc and the size of the inoculum, will have an impact on the size of the inhibition halo. Accordingly, the inoculum of the microorganism should be prepared in the form of a broth, which has been incubated for a period of between 4 and 6 hours, depending on the microorganism used, considering growth in exponential phase29.
The culture medium used has a profound impact on inhibition. For the majority of susceptibility tests, Müeller- Hinton agar has been used or another which is specially formulated which can be supplemented, if necessary, with blood or blood products, which do not have an effect on the activity of the majority of antimicrobial agents30.
In the disk-diffusion method used for fast-growing aerobic bacteria, the standard inoculum is distributed over the surface of a dish of Müeller-Hinton agar. Paper disks impregnated with antimicrobial agents are placed on the dish of agar. After incubation, the growth inhibition zone around each disk is measured and the results are compared with the guides published by the CSLI28.
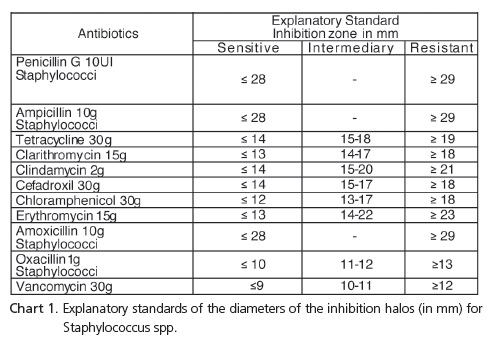
All media are affected by composition, pH, temperature and length of incubation. The incubation temperature for the majority of tests is 35-37ºC, for 18 to 24 hours, providing an excellent culture for the vast majority of human pathogens. The diameters of each agent's inhibition halos are interpreted as "sensitive", "resistant" or "intermediary". The term "sensitive" refers to a microorganism that responds to the test antibiotic, "resistant" to that in which therapy will probably be ineffective and "intermediary" to that where the microorganism only responds when high concentrations of the antimicrobial are achieved29.
Given the above, this study proposes to study the prevalence of strains of Staphylococcus aureus with regard to the materials used in the processing of intraoral x-rays and the level of resistance of these strains, through the Antibiotic Susceptibility test (AST).
METHODS
The present study was submitted to the Ethics in Research Committee of the São Leopoldo Mandic Faculty, which was approved under protocol no. 2009/0214.
Culture media
The following culture media were used: a) BHI agar (Brain Heart Infusion, Difco) - for the overall growth of the microorganisms and isolation of the colonyforming units (CFU); b) Mannitol salt agar (MSA, Difco) - for the differentiation of positive and coagulase-negative staphylococci; c) Müeller-Hinton agar (MHA, Oxoid) - for the performance of susceptibility tests.
Antibiotics
Paper disks were acquired for antibiograms (6.35mm, Cecon) impregnated with the following antibiotics: 1) ampicillin 10 μg; 2) chloramphenicol 30 μg; 3) erythromycin 15 μg; 4) clarithromycin 15 μg; 5) amoxicillin 10 μg; 6) oxacillin 1 μg; 7) penicillin G 10 UI; 8) clindamycin 2 μg; 9) tetracycline 30 μg; 10) vancomycin 30 μg; 11) cefadroxil 30 μg.
Assessment of contamination
Samples
The following samples were collected: Fifty lids from two boxes of portable dark rooms for radiographic processing, 50 samples (1ml each) of developing solution and 50 samples (1ml each) of fixing solution stored in the containers used for intraoral radiography processing. The samples were collected at the end of patient appointments at the Endodontic Clinic of the São Leopoldo Mandic Faculty in Campinas (SP), at the end of the day, during the period from February to December 2007.
Sample collection
After collecting the samples, 100 μL of each solution was placed in Eppendorf-type tubes containing 900 μL of sterile saline solution (NaCl 0.9%). The collections were made in duplicate for each sample. The tubes were then homogenized with the aid of a vortex type agitator and 10 μL of this solution was distributed on to Petri dishes containing 10 mL of BHI agar medium or 10 ml of mannitol salt agar. The dishes were incubated in a microbiological oven at 37ºC, for 24 hours. For each collection made, 4 tubes containing 900 μL of saline solution were used as the control group, and were subjected to the same aforementioned procedures.
Count of Staphylococcus aureus strains
After the period of incubation, the reading of the total number of colony-forming units (CFU) was taken. For the purposes of quantification, the manual counting technique was used, aided by a stereoscopic magnifier.
The results found, relating to the number of colonies, were expressed in CFU x 103 /ml, due to the sample having been diluted 1,000 times (initially a dilution of 10x on sample collection and then a dilution of 100x on the distribution of the sample on to the petri dishes for incubation).
Identification of colonies of Staphylococcus aureus
The identification of the microorganisms was based on analyses of the characteristics of colony growth, their morphology and via the Gram staining technique.
The following macroscopic characteristics were taken into consideration: colonies that were round, smooth, creamy, high, shiny or of a golden-yellow color. In a microscopic analysis, the presence was found of bacteria with a spherical format, generally arranged in irregular clusters and Gram-positive (purple coloration), as well as the growth in the selective medium of mannitol salt agar. In order to be able to differentiate between Staphylococcus aureus and Staphylococcus epidermidis, the coagulase test was carried out.
Antimicrobial Susceptibility test (AST)
Obtaining a concentration of 108 cfu/ml of the strains
After the microorganism was identified, as described in the previous item, the picking was performed of the colonies of S. aureus obtained that had been grown in the mannitol salt agar. These colonies (picking of 1 or 2 colonies) were inoculated in a BHI liquid medium and kept in microbiological ovens for a period of 4 hours in order to obtain an inoculum with a concentration of 108 cfu/ml.
In order to obtain a concentration of 108 cfu/ml, readings were taken in the spectrophotometer (model: Spectronic 20, make: Bausch & Lomb) of the test tubes containing BHI and bacteria until a bacterial suspension was obtained with an optic density of 60% transmittance, with the spectrophotometer previously set to zero with liquid BHI and adjusted to a wavelength of 800mm. The standardized inoculum was used to evaluate the resistance to antibiotics.
Evaluation of resistance to antibiotics
The standardized inoculum was administered on to dishes containing the medium Müller-Hinton agar with the aid of a sterile swab. The paper disks containing the antibiotics described above were then poured on to these inoculated dishes with the aid of tongs and were kept in a bacteriological culture oven at 37°C for 24 hours. Afterwards, the inhibition halos were measured (in millimeters) using a digital pachymeter (Starret® Brasil).
In order to evaluate resistance, the diameters of the inhibition halos were compared to those specified in the Table relating to the antibiotic susceptibility test standards published by the CLSI, using the following classification: sensitive, intermediary or resistant. Chart 1 shows the explanatory standards for inhibition halos.
RESULTS
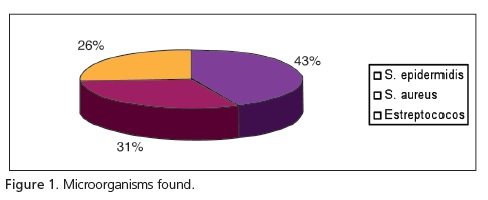
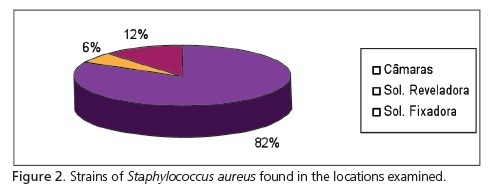
With regard to the microorganisms found, there was a greater prevalence of staphylococci (S. epidermidis - 43% and S. aureus - 31%) compared to streptococci (Figure 1). According to the results obtained, there was a greater prevalence of S. aureus in the portable dark room samples. In total, 52 strains of S. aureus were isolated, 43 having been obtained in the dark rooms, 3 from the developing solutions and 6 strains from the fixing solutions (Figure 2).
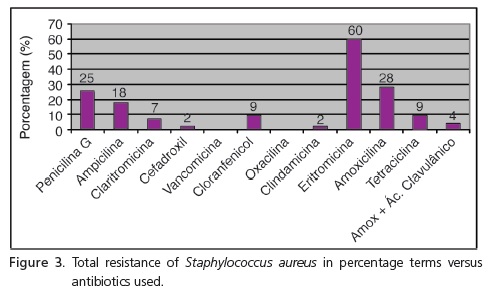
The Antibiotic Susceptibility Test (AST) was only performed for the samples that presented growth of S. aureus, confirmed by way of the growth of colonies in the Mannitol salt agar medium and identification through microscopic analysis of the colonies through Gram staining. As for the sensitivity to antibiotics, Figure 3 shows the percentage of bacterial resistance found for each antibiotic tested. Greater percentages for microorganism resistance were noted for Erythromycin and the antibiotics of the Penicillin group.
DISCUSSION
During the intraoral radiographic procedure, the radiographic films may be contaminated by oral microorganisms contained in patients' saliva or blood. Once contaminated and then handled without due care, they could contaminate the operator's hands and all places that he/she touches, such as: radiological equipment and materials used in the radiographic procedure. The potential for cross-contamination between patients through the handling by the professional of contaminated radiographic film has been demonstrated8.
This issue is aggravated by the fact that the majority of radiological clinics in Dental Faculties do not adopt procedures for the prior disinfection of x-ray apparatus and materials used in radiographic processing.
The present study demonstrated that there was contamination of the lids of the portable darkrooms and solutions used to develop the x-rays. These results may be attributed to a deficiency in the use of biosafety measures during radiographic processing.
S. aureus have been regarded as one of the most versatile and dangerous human pathogens, found in the oral cavity9. In addition to the capacity to survive on surfaces of different environments for a period of up to five days, these microorganisms have shown a worrying increase in resistance to antibiotics, such as methicillin and vancomycin.
Numerous studies in the literature have reported on the pathogenic potential of S. aureus in clinical environments, principally in hospitals13-17. In Dentistry, there have been few studies evaluating the level of resistance of this microorganism isolated from materials and equipment in dental clinics4-5. In Radiology, no studies at all have been found evaluating the levels of resistance of this microorganism when isolated from radiological equipment and materials.
In the present study, the place that presented the largest growth of bacteria was the lid of the portable darkrooms, with a prevalence of 82% S. aureus in the samples collected. This may be due to the fact that it is the area most handled by the professional who, when handling a potentially contaminated film and/or using contaminated gloves, promoted greater contamination of this area5.
The percentage of resistance of S. aureus was greater with antibiotics of the Penicillin group (penicillin G, ampicillin and amoxicillin) and erythromycin; having obtained a resistance percentage of 25%, 18%, 28%, and 60%, respectively. These results generated some concern as the antibiotics of the Penicillin group are used as the first option to treat mild to moderately severe infections.
There are basically two mechanisms responsible for resistance of S. aureus to the antibiotics of the Penicillin group. One mechanism is the production of betalactamases, enzymes responsible for the development of resistance to Penicillin. Another mechanism is an alteration in the penicillin binding proteins (PBPs). Moreover, resistance may be due to alterations in permeability, preventing the antibiotic from reaching its receptor.
Bacterial resistance to penicillin, caused by the production of beta-lactamase, can be avoided by the coadministration of a beta-lactamase inhibitor, clavulanic acid. Its function is to protect amoxicillin from degradation of the beta-lactamase enzymes and extend its spectrum of antimicrobial action by including bacteria resistant to amoxicillin and other beta-lactamase antibiotics16. In the current study, the percentage of resistance to amoxicillin was 28% and this percentage fell significantly when it was tested in association with clavulanic acid (4%), showing that the association was more effective on the tested microorganism.
Methicillin, a semi-synthetic derivative of penicillin, was the first antibiotic found to be stable in the presence of beta-lactamase. Oxacillin (isoxazolilic derivatives) was subsequently introduced to the market. The samples of S. aureus were seen to be sensitive to oxacillin and presented resistance to penicillin G.
As far as the macrolides are concerned, the samples presented resistance of just 7% to clarithromycin and 60% to erythromycin, demonstrating that erythromycin is an ineffective antimicrobial agent against this microorganism. This drug presents a spectrum of antibacterial action similar to that of penicillin G. Although considered to be an antibiotic with a small spectrum, it demonstrates activity against various microorganisms that are not affected by penicillin G27. The resistance to erythromycin (60%) found in this study was similar to that obtained by Tenover et al.19.
Clindamycin has an antimicrobial spectrum similar to that of erythromycin, with some exceptions: they have better activity against the majority of strains of S. aureus, and are most active against the majority of gram-positive and gram-negative anaerobes and possess a more restricted antibacterial spectrum that do not include Chlamydia, Rickettsia, Mycoplasma or the majority of Gram-negative aerobes27. S. aureus were only 2% resistant to clindamycin which demonstrates that this drug was more effective in comparison with erythromycin.
Chloramphenicol is an antibiotic with activity directed towards Gram-negative microorganisms, like some streptococci and staphylococci27. In this study, a figure of just 9% resistance to S. aureus to chloramphenicol was found.
A very small percentage (2%) of resistance to cefadroxil (cephalosporin) was also found, showing itself to be an effective antimicrobial against this microorganism. This medication is recommended for hospital use in patients with pneumonia and can be used by itself or in association with amoxicillin and clavulanic acid5-6.
Vancomycin is only used in medicine for the treatment of infections caused by gram-positive microorganisms sensitive to it and resistant to other more commonly used and less toxic antimicrobial drugs. Due to its less frequent use, many Gram-positive microorganisms that became resistant to other antibiotics, remained sensitive to vancomycin. The majority of strains of S. aureus, including strains resistant to methicillin remained sensitive to vancomycin27. In this study, no strain of the microorganisms obtained demonstrated resistance to vancomycin, in agreement with earlier studies4-5.
Although many studies in the literature may demonstrate a higher percentage of resistance of S. aureus, the results of the present study are also worrying, as the majority of dental clinics do not adopt biosafety measures with regard to radiological procedures. The absence of these procedures to control contamination could lead, in dental treatment, to the transmission of agents that cause disease in patients and professionals.
Accordingly, the adoption of contamination control procedures in Radiology is fundamental. The biosafety procedures used in Dentistry are practically the same as those used with other dental procedures.
The authors Sant'ana & Chinellato22 and Carvalho & Papaiz3 described certain safety measures which could be applied before, during and after taking the x-ray, and finally, during the development of the radiographic films.
According to these authors, some primary measures should be taken:
Before taking the x-ray: a) prior disinfection of the x-ray apparatus and places used to treat the patient; b) cover surfaces (materials and equipment) with waterproof plastic (PVC film, for example) and dispose of them after use; c) use PPE.
During the taking of the x-ray: a) use sterile materials and accessories; b) cover surfaces of radiographic films with waterproof plastic (PVC film); c) use PPE.
After taking the x-ray: a) remove the protective barrier (PVC film) or, if the barrier has not been used, remove excess saliva by washing the film and using a disinfectant on the film's protective cover.
During the development of the radiographic films: a) wear clean gloves after removing the waterproof plastic film and then carry out the film development stage. Though not mentioned by these authors, another measure that could contribute to avoiding contamination of the area used by the professional to carry out the radiographic procedure is the use of overgloves, which should be used when there is a risk of contamination via the gloves from other materials and places used by the professional in the procedure.
The adoption, in Radiology, of these safety measures that avoid or minimize the risk of contamination, added to the awareness of dental professionals and academics in terms of infection control, are necessary for the safety in dental clinics of patients and professionals alike, as they reduce the real risk of cross-infection during the taking and processing of x-rays3.
CONCLUSION
Following the study of the bibliography and based on the results obtained through the experiments conducted, it may be concluded that there has been contamination by S. aureus on the lids of the portable darkrooms and radiographic processing solutions, which occurred due to a deficiency in the use of biosafety measures during the radiographic process. The Antibiotic Susceptibility Test (AST) conducted just for the samples that presented growth of S. aureus, demonstrated that there was greater resistance to Erythromycin and the antibiotics of the Penicillin group, while the isolation of S. aureus with a level of resistance for the majority of antibiotics tested, demonstrates the need to use biosafety measures in radiological procedures, with the aim of avoiding cross-contamination.
Collaborators
RM SANTOS, FLM SANTOS, JC RAMACCIATO and JLC JUNQUEIRA took part in all stages of the research study.
REFERENCES
1. Puttaiah R, Langlais RP, Katz JO, Langland OE. Infection control in dental radiology. J Calif Dent Assoc. 1995;23(5):21-8. [ Links ]
2. Bachman CE, White JM, Goodis HE, Rosenquist JW. Bacterial adherence and contamination during radiographic processing. Oral Surg Oral Med Oral Pathol. 1990;70(5):669-73. doi: 10.1016/0030-4220(90)90420-W.
3. Carvalho PL, Papaiz EG. Controle de infecção em radiologia odontológica. Rev Assoc Paul Cir Dent. 1999;53(3):202-4.
4. Bernardo WL, Boriollo MF, Gonçalves RB, Höfling JF. Staphylococcus aureus ampicillin-resistant from the odontological clinic environment. Rev Inst Med Trop Sao Paulo. 2005;47(1):19-24. doi: 10.1590/S0036-46652005000100004.
5. Motta RH, Groppo FC, Bergamaschi CC, Ramacciato JC, Baglie S, de Mattos-Filho TR. Isolation and antimicrobial resistance of Staphylococcus aureus isolates in a dental clinic environment. Infect Control Hosp Epidemiol. 2007;28(2):185-90. doi: 10.1086/510867.
6. Andrade ED. Terapêutica medicamentosa em odontologia. 2ª ed. São Paulo: Artes Médicas; 2006.
7. Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135(4):429-37.
8. Bartoloni JA, Chariton DG, Flint DJ. Infection control practices in dental radiology. Gen Dent. 2003;51(3):264-71.
9. Rahmatulla M, Almas K, al-Bagieh N. Cross infection in the high-touch areas of dental radiology clinics. Indian J Dent Res. 1996;7(3):97-102.
10. White SC, Glaze S. Interpatient microbiological crosscontamination after dental radiographic examination. J Am Dent Assoc. 1978;96(5):801-4.
11. Lawson SR, Sauer R, Loritsch MB. Bacterial survival on radiographic cassettes. Radiol Technol. 2002;73(6):507-10.
12. Hardman PK, Tilmon MF, Taylor TS. Radiographic solution contamination. Oral Surg Oral Med Oral Pathol. 1987;63(6):733- 7.
13. Hardy KJ, Oppenheim BA, Gossain S, Gao F, Hawkey PM. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients' acquisition of MRSA. Infect Control Hosp Epidemiol. 2006;27(2):127-32.
14. Griffiths C, Lamagni TL, Crowcroft NS, Duckworth G, Rooney C. Trends in MRSA in England and Wales: analysis of morbidity and mortality data for 1993-2002. Health Stat Q. Spring 2004;(21):15-22.
15. Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, Length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166-74. doi: 10.1086/502522.
16. Huang SS, Datta R, Platt R. Risk of acquiring antibioticresistant bacteria from prior room occupants. Arch Intern Med. 2006;166(18):1945-51. doi: 10.1001/archinte.166.18.1945.
17. Cağatay AA, Ozcan PE, Gulec L, Ince N, Tugrul S, Ozsut H, et al. Risk factors for mortality of nosocomial bacteraemia in intensive care units. Med Princ Pract. 2007;16(3):187-92.
18. Bradley SF. Methicillin-resistant Staphylococcus aureus: longterm care concerns. Am J Med. 1999;106(5A):2S-10S. doi: 10.3201/eid1506.080195.
19. Tenover FC, McAllister S, Fosheim G, McDougal LK, Carey RB, Limbago B, et al. Characterization of Staphylococcus aureus isolates from nasal cultures collected from individuals in the United States in 2001 to 2004. J Clin Microbiol. 2008;46(9):2837- 41. doi: 10.1128/ JCM.00480-08.
20. Kurita H, Kurashina K, Honda T. Nosocomial transmission of methicillin-resistant Staphylococcus aureus via the surfaces of the dental operatory. Br Dent J. 2006;201(5):297-300. doi: 10.1038/sj.bdj.4813974.
21. Thomas LP, Abramovitch K. Infection control for dental radiographic procedures. Tex Dent J. 2005;122(2):184-8.
22. Sant'ana E, Chinellatto LEM. Avaliação da efetividade de soluções desinfetantes utilizadas para o controle de infecção cruzada em filmes radiográficos intrabucais. Rev Fac Odontol Bauru. 1997;5(3/4):37-44.
23. Coogan MM, Patel M, Mladenova D. Efficacy of three surface disinfectants for dental radiographic films and gloves. J Dent. 2004;32(5):385-9. doi: 10.1016/j.jdent.2004.01.009.
24. Koneman EW. Diagnóstico microbiológico. 5ª ed. São Paulo: Medsi; 2001.
25. Freitas VR, Picoli SU. A coloração de Gram e as variações na sua execução. NewsLab. 2007;82:124-8.
26. Brasil. Ministério da Saúde. Secretaria de Políticas de Saúde. Programa Nacional de DST e Aids. Técnica de coloração de GRAM [texto na internet]. 2001. [citado 2010 Out 10]. Disponível em: <htpp://bvsms.saúde.gov.br/bvs/publicações>.
27. Montgomery EH. Antibióticos antibacterianos. In: Neidle EA, Yagiela JA. Farmacologia e terapêutica para dentistas. 4ª ed. Rio de Janeiro: Guanabara Koogan, 1998. p.469-502.
28. Medeiros EAS, Stempliuk VA, Santi LQ, Sallas J. Uso racional de antimicrobianos para prescritores. São Paulo: Unifesp; 2008.
29. Piddock LJ. Techniques used for the determination of antimicrobial resistance and sensitivity in bacteria. Antimicrobial Agents Research Group. J Appl Bacteriol. 1990;68(4):307-18.
30. Brenner VC, Sherris JC. Influence of diferent media and bloods on the results of diffusion antibiotic susceptibility tests. Antimicrob Agents Chemother. 1972;1(2):116-22.
 Correspondence to:
Correspondence to:
RM SANTOS
e-mail: rachelmedeiros@gmail.com
Received on: 15/9/2010
Final version resubmitted on: 25/10/2010
Approved on: 17/1/2011













