Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.4 Porto Alegre Out./Dez. 2012
ORIGINAL / ORIGINAL
Fluoride release from orthodontic composites: in vitro study
Liberação de flúor de compósitos ortodônticos: estudo in vitro
Rogério Lacerda SANTOS I; Gêisa Aiane de Morais SAMPAIO II; Matheus Melo PITHON III; Delmo Santiago VAITSMAN IV
I Universidade Federal de Campina Grande, Centro de Saúde e Tecnologia Rural, Faculdade de Odontologia, Departamento de Ortodontia. Patos, PB, Brasil
II Universidade Federal de Campina Grande, Centro de Saúde e Tecnologia Rural, Faculdade de Odontologia. Patos, PB, Brasil
III Universidade Estadual do Sudoeste da Bahia, Faculdade de Odontologia, Departamento de Ortodontia, Jequié, BA, Brasil
IV Universidade Federal do Rio de Janeiro, Departamento de Química. Rio de Janeiro, RJ, Brasil
ABSTRACT
Objective
To evaluate the behavior of orthodontic composites, given their fluoride release and uptake ability.
Methods
The materials were divided into 3 groups: 2 orthodontic composites and 1 resin-reinforced glass ionomer cement used for bonding orthodontic brackets, as follows: Group Q (Quick Cure, Reliance), Group A (Aqualite, OrthoSource) and Group OGLC (Ortho Glass LC, DFL) and a control group. The fluoride release was measured for 28 days (after 1 hour and then 1, 3, 7, 14, 21 and 28 days) via an ion-selective electrode connected to an ion analyzer. To evaluate the recharge of fluoride, the specimens were exposed to sodium fluoride solution (1,000 ppm fluoride) for 28, 30, 31 and 32 days. The variance analysis (ANOVA) and Tukey's tests were used to evaluate between groups (p< 0.05).
Results
The results showed that the materials reached the peak of fluoride release 24 hours after polymerization. There was a statistical difference between groups Q and A at the time intervals of 1 hour and at at 1, 3, 7, 14 days and after recharge with fluoride (p˂ 0.05). The Q and A groups showed a statistical difference with the Group OGLC in all periods p< 0.05).
Conclusion
The Quick Cure composite showed a higher initial ability for fluoride uptake and release compared to the Aqualite composite. Both composites showed similar performance after 21 dias.
Indexing terms: Composite resins. Fluorine. Sodium fluoride.
RESUMO
Objetivo
Avaliar o comportamento de compósitos ortodônticos, quanto a capacidade de liberação e captação de flúor.
Métodos
Os materiais foram divididos em 3 grupos: 2 compósitos ortodônticos e 1 cimento de ionômero de vidro reforçado com resina utilizados para colagem de bráquetes ortodônticos, sendo: Grupo Q (Quick Cure, Reliance), Grupo A (Aqualite, OrthoSource) e Grupo OGLC (Ortho Glass LC, DFL), como controle. A liberação de flúor foi medida durante 28 dias (1h e 1, 3, 7, 14, 21 e 28 dias), através de um eletrodo íon seletivo conectado a um analisador de íons. Para avaliação da recarga de flúor, os espécimes foram expostos a solução de fluoreto de sódio (1000 ppm de flúor) nos dias 28, 30, 31 e 32. A análise de variância (ANOVA) e teste de Tukey foram utilizados para avaliação entre grupos (p< 0.05).
Resultados
Os resultados evidenciaram que os materiais atingiram o pico máximo de liberação de flúor com 24 horas após a polimerização. Houve diferença estatística entre os grupos Q e A nos tempos de 1 hora , 1, 3, 7, 14 dias e após recarga com flúor (p<0.05). Os grupos Q e A apresentaram diferença estatística com o grupo OGLC em todos os períodos (p<0.05).
Conclusão
O compósito Quick Cure apresentou maior capacidade de liberação e captação de flúor inicial comparado ao compósito Aqualite. Ambos os compósitos apresentaram desempenho semelhante após 21 dias.
Termos de indexação: Resina composta. Flúor. Fluoreto de sódio.
INTRODUCTION
The demineralization of tooth enamel is common in patients subjected to fixed orthodontic treatments which present substandard oral hygiene1. In Orthodontics, white spot lesions have been the motivation for a number of studies1, and the search for materials that could prevent such damage, is a concern at the present time1-2.
Fluoride's cariostatic action mechanism occurs via the inhibition of demineralization and an increase in remineralization of the affected area3. In addition, the bactericidal and antienzymatic action of the sodium fluoride has been reported4. Given this, the application of topical fluoride and/or the use of fluoride rinses linked with oral hygiene can help to reduce demineralization of the enamel adjacent to the orthodontic brackets5.
Amongst orthodontic materials, composites are widely used and their capacity to release fluoride1 over long periods has been reported, as well as the potential to minimize tooth demineralization3. Nevertheless, the quantity of fluoride released from the resin composites is small when compared to ionomer materials3,5.
Given the importance of carrying out studies on the release of fluoride from orthodontic materials, it was the authors' aim to evaluate the behavior of orthodontic composites with regard to their capacity for fluoride uptake and release.
METHODS
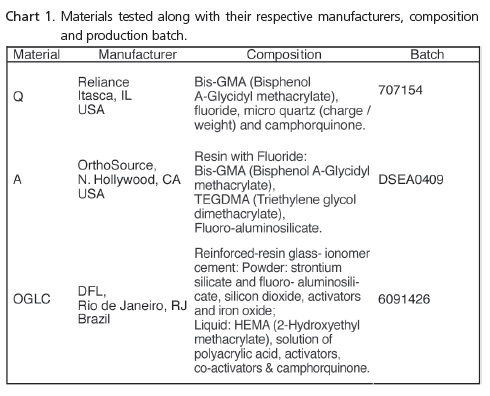
Three materials used for bonding orthodontic accessories were evaluated: 2 composites, Group Q (Quick Cure, Reliance, Itasca, IL, USA); Group A (Aqualite, OrthoSource, N. Hollywood, CA, USA) and 1 resin-reinforced glass ionomer cement, Group OGLC (Ortho Glass LC, DFL, Rio de Janeiro, Brazil), as a control group (Chart 1).
A total of 30 test specimens were produced, 10 for each material, using silicon molds with a diameter of 4 mm and height also of 4mm. The glass ionomer cement was handled in accordance with the manufacturer's instructions, by one operator only. The materials were inserted into the molds with the aid of a syringe (Centrix, DFL, Rio de Janeiro, Brazil) in order to avoid the formation of bubbles. The surface of the material was covered with a glass slide using finger pressure, thus flattening out its surface, and then the photopolymerization was carried out (Radii, SDI, Bayswater, Victoria, Australia) according to the manufacturer's instructions. The test specimens were kept this way this for 10 minutes and were then stored for 30 minutes at 37ºC and a humidity of 100%. At the end of this interval, two specimens were placed in 8 ml of deionized water by means of a Milli-Q purification system (Millipore, Bedford, MA, USA) in a glass container and maintained in an oven at 37ºC. Every 24 hours, the specimens were lightly dried with absorbent paper towels and the water in each container was replaced. This procedure was followed in order to avoid a buildup of fluoride, as reported by Kuvvetli et al.6.
The solutions of 8 ml and 2 ml of deionized water used to wash the specimens were mixed and diluted 5 times and adjusted with 50 ml of total ionic strength adjustment buffer (TISAB). The fluoride concentrations were analyzed by combining an ion-selective electrode (Thermo Orion 9609, Orion Research Inc., Boston, MA, USA) and an ion analyzer (pH/ion, 450M Analyzer, São Paulo, SP, Brazil). The electrode was calibrated on a daily basis, with standard solutions of 0.05, 0.1 and 0.19 ppm of fluoride, throughout the study. The concentrations of fluoride released from each material were measured and the data were transformed into μg/cm² to verify the amount of fluoride released by the area of the test specimen. The release of fluoride was measured after 1 hour and then at 1, 3, 7, 14, 21 and 28 days.
At the end of 28 days, the specimens were washed in deionized water for 20 seconds and the surface was lightly dried using disposable, absorbent paper towels. The specimens were then immersed in a solution of sodium fluoride 0.221% (1,000 ppm of fluoride) on day 28 for 5 minutes and were subsequently washed with deionized water for 20 seconds. Two specimens were placed in 8 ml of deionized water in a glass container and the fluoride release was measured after 24 and 48 hours (days 29 and 30) to observe the timing of the release of the absorbed fluoride. On days 30, 31 and 32, new fluoride recharges were performed as described above and the fluoride release was measured after 24 hours (days 31, 32 and 33) to observe the ability to retain the recharge.
The variance analysis (ANOVA) and Tukey test were used for the evaluation between groups, with reliability at a significance level of 0.05 for the identification of the statistical difference in fluoride release.
RESULTS
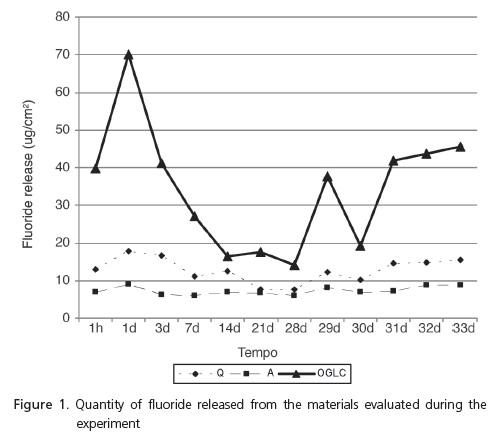
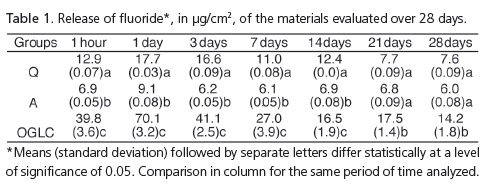
The materials reached the maximum peak of fluoride release 24 hours after polymerization (Figure 1). There was a statistical difference between groups Q and a at the intervals of 1 hour and at 1, 3, 7 and 14 days (p˂ 0.05) (Table 1). Groups Q and A demonstrated a statistical difference with group OGLC at all the time intervals (p< 0.05) (Tables 1 and 2).
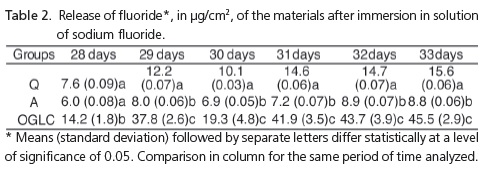
On day 29, the first day after the fluoride recharge, all the groups showed higher fluoride release, with a statistical difference between the groups (p= 0.001) (Table 2). On day 30, which corresponds to 48 hours after the first fluoride recharge, there was a reduction in fluoride release; there was a statistical difference between the groups (p= 0.001). On days 31, 32 and 33, 24 hours after the last fluoride applications (days 30, 31 and 32), it was noted that the group OGLC had the highest capacity for fluoride uptake and release, followed by groups Q and A; there was a statistically significant difference between the 3 groups (p< 0.05) (Table 2).
DISCUSSION
Patients with fixed orthodontic appliances7 are more susceptible to tooth demineralization adjacent to the orthodontic bracket, due to the greater retention of food and to substandard hygiene2,5,8-10. So the use of adhesive materials for orthodontic accessories that have the ability to release fluoride is essential in helping to prevent such demineralization.
Various fluoridated materials have been developed for orthodontic use, including glass-ionomer cements, resin-modified glass-ionomer cements (RMGIC), polyacidmodified resin composites (compomers) and composites. Many studies1,3,5-6,8-9 have shown that these materials have specific fluoride and the ability to release this fluoride11.
Amongst these materials, composites are widely used in orthodontics12, and serve as the main reservoir of fluoride ions at the periphery of the orthodontic accessories13, however improvement is needed in terms of their compositions, for the effective prevention of white spot lesions12. Therefore the objective of the authors was to compare and contrast the performance of orthodontic composites with regard to the release of fluoride.
The concentration of the fluoride solution used was 1,000 ppm fluoride, as it is similar to the fluoride concentration present in the majority of dentrifices used for oral brushing8-10,14. Deionized water was used in this study as it does not have any ions that could have an impact on results8 and the water was replaced daily to avoid a buildup of fluoride in the solution3,8-10.
The use of silicon molds 4 mm high by 4 mm in diameter was based on other studies8-10.
The results showed that the release of fluoride was present in all the materials tested and in all periods of the study, and was greatest 24 hours after the initial adhesion, decreasing by days 3, 7 and 14, and with very little variation after the 14th day. These results are similar to other orthodontic cements in similar studies2,8-10,14.
The release of fluoride from the composite Aqualite was lower than with the other two materials, however its release could be detected throughout the duration of the experiment, which is significant for the effective prevention of demineralization of the enamel around the orthodontic appliances15-16. The behavior of the release of fluoride from the composites Quick-cure and Aqualite is explained by the composition of these materials, by the presence of fluoridated monomer and fluoro-aluminosilicate respectively. The amount of fluoride released from these materials is related to the form of chemical combination and/or the quantity of intrinsic fluorine17.
The Ortho Glass LC RMGICs demonstrated the best performance during the experiment. This result was expected since the RMGICs have a more porous surface than the composites and this porosity permits a mechanism of greater diffusion of the fluoride recharge, which results in a greater quantity of storage and release of this ion6.
Studies8-10 have confirmed that the exposure of adhesive materials to the solution of sodium fluoride leads to the uptake and release of fluoride, shown by the increase in fluoride release in the days subsequent to the recharge11.
In this study, the materials with the highest levels of initial fluoride release had a similar performance after the fluoride recharge, which demonstrates the greater uptake and release capacity of this ion8-10 by the materials.
All the materials demonstrated fluoride release in excess of 5 μg/cm² of fluoride, at all the time intervals of the study which, according to Ten Cate et al.18, is a concentration sufficient to prevent the demineralization of the dentin, however, when faced with patients with a high risk of caries, the composite Quick-cure is the most recommended.
In order to prevent white spot lesions adequately, in addition to the release of fluoride from the adhesive materials, the need for appropriate oral hygiene using fluoridated dentrifice11 should be emphasized as well as a periodic checkup by the orthodontist or GP, as the released fluoride may not adequately prevent or inhibit demineralization adjacent to the brackets2,8-10.
CONCLUSION
The evaluated composites have different initial fluoride uptake and release capacity, however they do perform similarly after 21 days of evaluation.
Collaborators
RL SANTOS participated in the creation, preparation and composition of the article. GAM SAMPAIO and MM PITHON participated in the production and composition of the article. DS VAITSMAN directed the research study and took part in the composition of the article.
REFERENCES
1. Todd MA, Staley RN, Kanellis MJ, Donly KJ, Wefel JS. Effect of a fluoride varnish on demineralization adjacent to orthodontic brackets. Am J Orthod Dentofacial Orthop. 1999;116(2):159- 67. [ Links ]
2. Pithon MM, Oliveira MV, Santos RL, Bolognese AM, Ruellas ACO. Avaliação in vitro da resistência ao cisalhamento e liberação de flúor de dois cimentos de ionômero de vidro reforçado por resina. Rev Odonto Ciênc. 2007;22(58):305-10.
3. Chin MYH, Sandham A, Rumachik EN, Ruben JL, Huysmanseet MCD. Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthod Dentofacial Orthop. 2009;136(4):547-53. doi: 10.1016/j. ajodo.2007.10.053.
4. Chitnis D, Dunn WJ, Gonzales DA. Comparison of in-vitro bond strengths between resin-modified glass ionomer, polyacidmodified composite resin, and giomer adhesive systems. Am J Orthod Dentofacial Orthop. 2006;129(3):11-6. 10.1016/j. ajodo.2005.11.011.
5. Cacciafesta V, Sfondrini MF, Tagliani P, Klersyd C. In-vitro fluoride release rates from 9 orthodontic bonding adhesives. Am J Orthod Dentofacial Orthop. 2007;132(5):656-62. doi: 10.1016/j.ajodo.2005.09.037.
6. Kuvvetli SS, Tuna EB, Cildir SK, Sandalli N, Gencay K. Evaluation of the fluoride release from orthodontic band cements. Am J Dent. 2006;19(5):275-8.
7. Tufekci E, Dixon JS, Gunsolley JC, Lindauer SJ. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011;81(6):206-10. doi: 10.2319/051710-262.1.
8. Santos RL, Pithon MM, Leonardo JBP, Oberosler ELC, Vaitsman DS, Ruellas ACO. Liberação de flúor de cimentos ortodônticos antes e após recarga com solução fluoretada. Rev Odonto Ciênc. 2009;24(1):54-8.
9. Santos RL, Pithon MM, Vaitsman DS, Araújo MTS, Souza MMG, Nojima MGC. Long-term fluoride release from resinreinforced orthodontic cements following recharge with fluoride solution. Braz Dent J. 2010;21(2):98-103. doi: 10.1590/S0103- 64402010000200002.
10. Leonardo JBP, Santos RL, Vaitsman DS, Sant'anna EF, Ruellas ACO. Liberação de flúor de cimentos ortodônticos com e sem proteção imediata. Rev Bras Odontol. 2008;65(2):280-4.
11. Ahn SJ, Lee SJ, Lee DY, Lim BS. Effects of different fluoride recharging protocols on fluoride ion release from various orthodontic adhesives. J Dent. 2011;39(3):196-201. doi: 10.1016/j.jdent.2010.12.003.
12. Vilchis RJS, Yamamoto S, Kitai N, Hotta M, Yamamoto K. Shear bond strength of a new fluoride-releasing orthodontic adhesive. Dent Mater J. 2007;26(1):45-51. doi: 10.4012/dmj.26.45.
13. Fonseca DDD, Costa DPTS, Simões R, Beatrice LCS, Araújo ACS. Adesivos para colagem de braquetes ortodônticos. RGO - Rev Gaúcha Odontol. 2010;58(1):95-102.
14. Okuyama K, Murata Y, Pereira PN, Miguez PA, Komatsu H, Sano H. Fluoride release and uptake by various dental materials after fluoride application. Am J Dent. 2006;19(2):123-7.
15. Pithon MM, Bernardes LAA, Ruellas ACO, Romano FL. Avaliação da resistência ao cisalhamento do compósito Right- On em diferentes condições de esmalte. Rev Dental Press Ortodon Ortop Facial. 2008;13(3):60-5. doi: 10.1590/S1415- 54192008000300008.
16. Pithon MM, Oliveira VM, Sant'anna EF, Ruellas ACO. Avaliação da resistência ao cisalhamento do compósito eagle bond. Rev Saúde.com. 2007;3(2):3-9.
17. Lim BS, Lee SJ, Lim YJ, Ahn SJ. Effects of periodic fluoride treatment on fluoride ion release from fresh orthodontic adhesives. J Dent. 2011;39(11):788-794. doi: 10.1016/j. jdent.2011.08.011.
18. Ten Cate JJ, Damen JJM, Buijs MJ. Inhibition of dentin demineralization by fluoride in vitro. Caries Res. 1998;32(2):141- 7. doi: 10.1159/000016444.
 Correspondence to:
Correspondence to:
RL SANTOS
e-mail: lacerdaorto@hotmail.com
Received on: 26/9/2011
Final version resubmitted on: 9/2/2012
Approved on: 18/2/2012













