Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.4 Porto Alegre Out./Dez. 2012
ORIGINAL / ORIGINAL
Immunolocalization of FGF-10 in murine incisors and molar germs
Imunolocalização de FGF-10 em germes de incisivos e molares murinos
Jussara Peixoto ENNES I; Vanessa Soares LARA II; Inês Aparecida TOZETTI I; Daniella Moraes ANTUNES I; Gabriell Bonifácio BORGATO I
I Universidade Federal de Mato Grosso do Sul, Centro de Ciências Biológicas e da Saúde. Campo Grande, MS, Brasil
II Universidade de São Paulo, Faculdade de Odontologia, Departamento de Estomatologia. Bauru, SP, Brasil
ABSTRACT
Objective
The aim of this study was to identify the presence of FGF-10 in mouse dental germs by means of the immunohistochemical technique, from the initial development phase through to the more advanced phases.
Methods
Fetuses of five mice, on days 15.5, 16.5, 17.5, 18.5 and 19.5 of pregnancy, respectively, were collected. At time intervals of 0.5, 1.5, 2.5 and 3.5 days after birth, the mouse offspring were sacrificed. The heads of all the specimens were fixed and submitted to histotechnique and 3μm thick sections were obtained. The presence of FGF-10 was detected by means of the avidin-biotin-peroxidase immunohistochemistry technique.
Results
Immunostaining was detected in both epithelium and ectomesenchyme with intensity and spatial-temporal differences. A reduction in the presence of FGF-10 was observed in the cervical loop area, on the lingual side of the incisor crowns, and both sides of the molar crowns. With the increase in enamel matrix deposition, immunostaining on the secretory pole of ameloblasts also increased.
Conclusion
FGF-10 immunostaining could be related to cell proliferation in epithelium and cell differentiation in epithelium and ectomesenchyme. This could be related to morphological determination in intercuspal areas of molar germs and to continuous growth of the incisor crown. Decrease in FGF-10 in the cervical loop could be related to the termination of crown formation. Increase in FGF-10 expression in ameloblasts suggests a relationship with active enamel production. The results suggest the inclusion of the pattern of the presence of FGF-10 in future investigations into the cause of morphological anomalies, such as palato-gingival groove.
Indexing terms: Immunohistochemistry. Odontogenesis. Tooth development.
RESUMO
Objetivo
Identificar a presença de FGF-10 em germes dentários de rato pela técnica de imunohistoquímica.
Métodos
Os fetos de cinco ratos, nos dias 15,5; 16,5; 17,5; 18,5; 19,5, respectivamente, de gravidez, foram coletados. Em intervalos de tempo de 0,5; 1,5; 2,5 e 3,5 dias após o nascimento, as proles de ratas foram sacrificadas. As cabeças de todos os exemplares foram fixadas e submetidas à histotécnica e cortes com 3μm de espessura foram obtidos. A presença de FGF-10 foi detectada pela técnica de imunohistoquímica da avidinabiotina- peroxidase.
Resultados
A imunomarcação foi detectada no epitélio e no ectomesênquima com intensidade e diferenças espaço-temporais. Uma redução da presença de FGF-10 foi observada na área da alça cervical, no lado lingual de coroas de incisivo e em ambos os lados das coroas de molares. Com o aumento da deposição de matriz de esmalte, a imunomarcação no pólo secretor de ameloblastos também aumentou.
Conclusão
A presença de FGF-10 parece estar relacionada com a proliferação de células no epitélio e diferenciação de células no epitélio e ectomesênquima. Isso pode estar relacionado à determinação morfológica nas áreas intercuspídeas de germes dos molares e ao crescimento contínuo da coroa nos incisivos. A diminuição do FGF-10 em áreas alça cervical parece estar relacionada com o término de formação da coroa. O aumento de FGF-10 em ameloblastos parece estar relacionada com a produção ativa de esmalte.
Termos de indexação: Imunoistoquímica. Odontogênese. Germe de dente.
INTRODUCTION
Odontogenesis is the result of a manifestation of an polygenic system, in which the development genes manifest themselves in a sequential, cyclic pattern1-2. Over 200 genes have been associated with odontogenesis1-2 and dental defects1,3.
Many molecules and some gene cascades have been identified in the initial phases of tooth development: bud, cap, bell, and to a lesser degree, root formation4-10. Families of molecules such as those of Wnt, Hh (hedgehog), FGFs (Fibroblast Growth Factor) and BMPs (Bone Morphogenetic Protein, of TGFßs - Transforming Growth Factor family) have been identified, in different proportions and sites, with tissue-specific and stage-specific functions1-2. They have been related to functions such as determining the fate of neural crest cells during tooth morphogenesis11, stimulating interactions between the ectoderm and ectomesenchyme (cell induction, proliferation, condensation and differentiation); forming signaling centers, as well as inducing transcription factors2. Disturbances in gene expression patterns have been related to tooth anomalies such as tooth agenesis, supernumerary teeth and root malformation1,3. All these studies have brought knowledge that has led to achievements in bio dental engineering2,12-14.
FGFs expression, is associated with initiation eventsinducing the expression of other genes2 by stimulating cell proliferation in the epithelium15-16. The expression of FGF-10 would thus maintain a stem cell compartment17-18 that would supply epithelial cells to the inner dental epithelium, which would remain as cells of the stratum intermediate18 or would differentiate into ameloblasts15. The cessation of FGF-10 expression would interrupt crown formation and lead to root development4,8,17. Its expression has also been related to tooth morphogenesis as it would induce epithelial cell proliferation1,15.
FGF-10 has been related to cell proliferation15,19-21 and fate15, in early tooth developmental phases. The pattern of FGF-10 presence, from early to later stages of tooth development, might help us to understand the relationship between this molecule and events in tooth development. In addition, its distribution pattern might help with the understanding of anomalies of tooth morphology in future approaches. The aim of this study was to investigate the presence of FGF-10 in incisor and molar germs in mice, from the initial embryonic days of development (E): E15.5, E16.5, E17.5, E18.5, E19.5 to the more advanced phases 0.5, 1.5, 2.5 and 3.5 days after birth.
METHODS
The experiment was approved by the UFMS Ethics Committee on the Use of Animals, Protocol No. 159/07. Five female mice were anesthetized, on days 15.5, 16.5, 17.5, 18.5 and 19.5 of pregnancy, respectively, using a combination of Ketalar® (0.08ml/100g), Virbaxil® (0.04/100g), prior to the caesarean section, when the fetuses were collected. At time intervals of 0.5, 1.5, 2.5 and 3.5 days after birth, the mouse offspring were sacrificed. Their heads were fixed in 10% formaldehyde for 48 hours and submitted to histotechnique, embedded in paraffin, alternating their sectional planes: the first was in the front section plane and the second was in the sagittal plane. The sections, about 3μm thick, were applied to silanized slides.
The presence of FGF-10 was detected by the avidin-biotin-peroxidase immunohistochemistry technique19. After removing the paraffin from the sections with xylol and rehydrating them through a series of graded ethanol baths, antigen recovery was performed through humid heat treatment in citrate buffer for two and a half minutes. After cooling for 20 minutes, endogen peroxidase was blocked by 10 volume-H2O2 baths of 10 minutes each. Next, the sections were washed with distilled water, neutralized with Phosphate Buffered Saline (PBS) and dried thoroughly. The slides were incubated with rabbit anti-mouse antibody FGF-10 (200μg/ml, H-121, Santa Cruz Biotechnology) diluted in PBS and albumin at 1/100 concentration for 18 hours, at 4ºC. After being washed with PBS, the sections were thoroughly dried in order to receive a pre-prepared solution of biotinylated antibodies for an exposure of 40 minutes. Before being exposed to streptavidin for 30 minutes, the slides were properly washed and dried and were stained with chromogenic diaminobenzidine (DAB) solution and incubated for 30 minutes. After being washed, the sections were quickly stained with hematoxylin, washed and passed through ammonia water, dehydrated through a series of graded ethanol baths, immersed in xylol to be assembled with Entellan.
Positive control of the reactions was obtained using rabbit ovary sections; and the negative control was obtained by replacing the primary antibodies with PBS-BSA 1%.
RESULTS
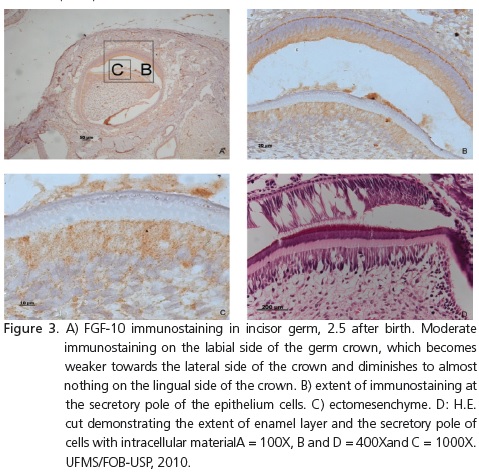
The FGF-10 immunostaining pattern in incisor germs is summarized in Chart 1.
In early incisor crown development, at E15.5, the cap phase could be identified. Immunostaining was evident in the proximal pole of inner epithelium cells. In the ectomesenchyme, it was identified in the cells facing the basal membrane and also between more distant cells.
Both E16.5 and E15.5 incisor germs presented very similar immunostaining patterns, with moderate immunostaining in both tissues. As the cells of the inner epithelium were proliferating, staining was more intense and covered a larger area of cytoplasm in their secretory pole. Epithelial cells also presented immunostaining in the distal pole, although less intensely. Longitudinal sections showed a predominance of moderate immunostaining of epithelial and ectomesenchymal cells on the labial side of the tooth germ crown. The ectomesenchymal cells that were condensed and turned towards the epithelium presented more intense immunostaining.
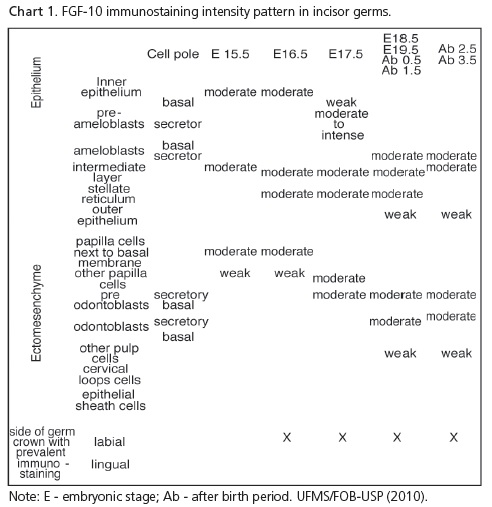
At E17.5, incisors were in the bell stage. In the ectomesenchyme, moderate immunostaining was observed in the secretory poles of more differentiated cells (Figure 1A) and in non-differentiated cells of the papilla, further away from the enamel organ. On the proximal side of the transverse sections of these incisor germs, weak immunostaining could be observed, predominantly in epithelial cells (Figure 1B), while weaker on the lingual side; almost no immunostaining was seen (Figure 1C). In longitudinal sections, the predominance of FGF-10 immunostaining distribution could be followed all the way through the labial side of the developing crown (Figure 1D). This could also be observed in the other periods analyzed: E18.5, E19.5, 0.5, 1.5, 2.5 (Figure 2) and 3.5 days after birth.
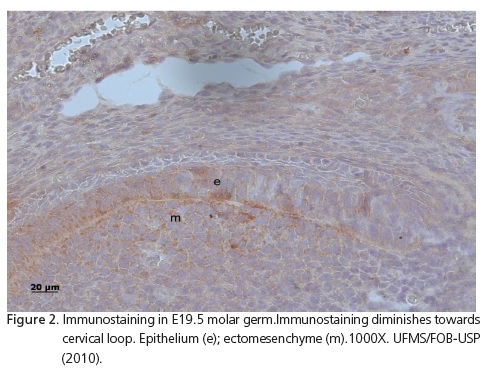
In the development of incisor germs at E18.5E, 19.5E, 0.5, 1.5, 2.5 and 3.5 days after birth, immunostaining followed the pattern observed in the previous phases. The outer epithelium cells also presented immunostaining in the distal pole, facing the dental follicle. As the enamel layer increased, so did the extent of immunostaining at the secretory pole of the ameloblasts (Figure 3).
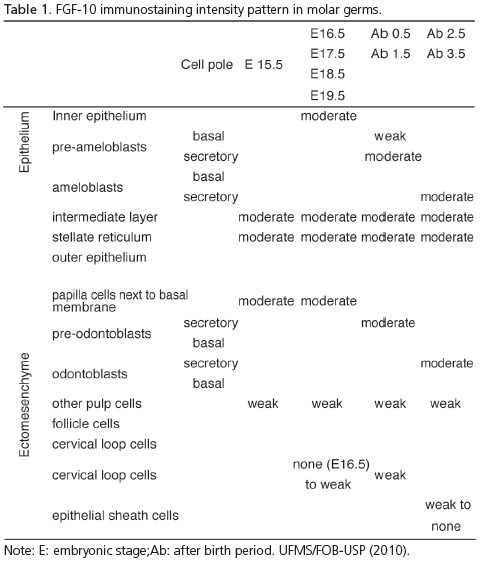
TheFGF-10 immunostaining pattern in molar germs is summarized in Table 1.
In molar development at E15.5, the early cap stage structure was typically organized. In maxillary and mandibular molar crowns, at E16.5, development of the same pattern of immunostaining observed in E15.5 molar germs was identified.
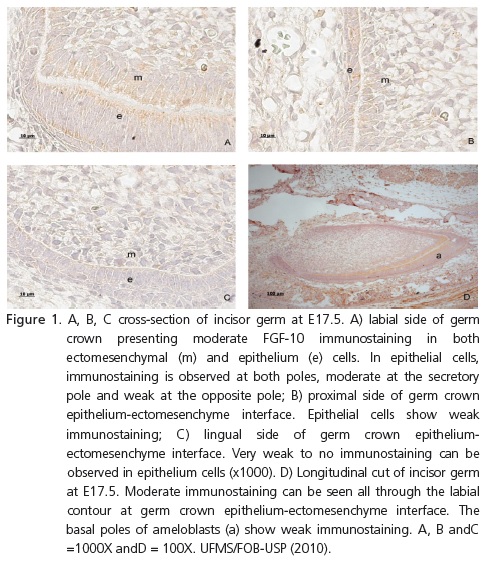
Development of the maxillary and mandibular molar crowns at E17.5, 18.5 and 19.5 presented a very similar immunostaining pattern. The folding of inner epithelium (enamel knots) could be observed in areas related to the localization of future cusps. In areas related to the cervical loop, immunostaining diminished in both the epithelium and ectomesenchyme (Figure 2).
After birth, on days 0.5 and 1.5, the first maxillary molar germ presented a similar pattern (Table 2).
After birth, on days 2.5 and 3.5, molar sections presented a similar pattern with moderate immunostaining in both ameloblasts and odontoblasts at the secretory pole. The length of the secretory pole presenting staining grew with the increase in enamel deposition (Figure 4).
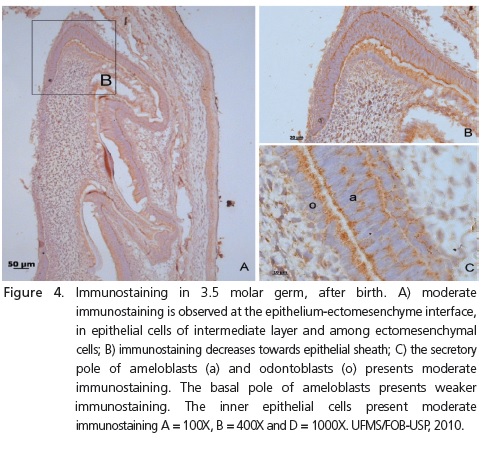
DISCUSSION
In the present study, immunolocalization of FGF-10 was observed in incisor and molar germs in all the phases analyzed, in both the epithelium and ectomesenchyme, with a variation in intensity and localization in these phases.In this study, FGF-10 was present in areas related to epithelial and ectomesenchymal cell proliferation in the early developmental stages, (E15.5; E16.6) as previously reported15; and in cell differentiation areas (from E17.5 to day 3.5 after birth) in incisors and molars. The expression of FGF- 10 has been identified in the mesenchyme, and is related to epithelialstem cell proliferation, maintenance and fate8,17-18,20-21. Other studies have described the presence of FGF-10 only in the ectomesenchyme and related to epithelium cell proliferation15,21 and differentiation15. The immunostaining intensity observed in the ectomesenchyme was higher in the area at the epithelial-mesenchymal interface than in the remaining part of the papilla. FGF-10 expression in the mesenchyme has previously been reported and is related to the induction of cell proliferation in the epithelium15,18,20.
In longitudinal sections of the incisor, immunostaining in both the ectomesenchyme and epithelium could be seen. It occurred predominantly on the labial side of the crown, where pre-ameloblasts and pre-odontoblasts could be observed, as previously reported18. Very weak to no immunostaining was observed in the epithelium of the crown on the lingual side. FGF-10 expression was related to the maintenance of an adult epithelium stem cell for crown formation4,8. Thus, the presence of FGF-10 on the labial side of incisor crowns, observed in all periods analyzed, could be associated with continuous tooth growth which demands crown development with enamel deposition on the labial side of the crown. In molars, moderate FGF- 10 immunostaining was also observed in the stellate reticulum and inner epithelium, from E15.5 through all other periods analyzed. These areas were related to stem cell proliferation and maintenance and to ameloblast precursor cell proliferation, respectively20, while the crown was forming. At E17.5, epithelial cells presented moderate immunostaining in intercuspal areas and in the stellate reticulum. Cell proliferation is necessary to promote growth and is also associated with morphological determination. Areas of cell proliferation around areas in which cell apoptosis is identified are related to cusp determination2. The determination of some morphological tooth anomalies could also involve these events, but with some disturbances of localization or intensity in gene expression cascades. FGF-10 deficient mice fail to form a cervical loop and present interruption of dental epithelial development in their explants17. Therefore, studies on palato-gingival groove, for example, should explore the pattern of FGF-10 presence.
The extent of FGF-10 immunostaining increased at the secretory pole of ameloblasts, as the extent of the enamel layer increased.The presence of FGF-10 has previously been related to epithelial cell fate15,18,21, but this study showed a later stage of tooth development, up to 3.5 days after birth, when FGF-10 may have some relationship with cell activity, such as active enamel production.
In molars, FGF-10 presented moderate immunostaining at E15.5, E16.5in mesenchymal cells. This could be related to cell proliferation areas, such as areas of enamel knots, as previously reported2,15.
CONCLUSION
Immunostaining of FGF-10 was identified in both the epithelium and ectomesenchyme in all tooth germ stages analyzed and could be associated with cell proliferation in the epithelium and cell differentiation in the epithelium and ectomesenchyme. It could be related to morphological determination in the intercuspal areas of molar germs and to continuous growth of the crown in incisors. A decrease in the presence of FGF-10 was observed in the cervical loop area on the lingual side of the incisor crown and on both sides of molar crowns. This could be related to the termination of crown formation. With the increase in enamel matrix deposition, immunostaining at the secretory pole of ameloblasts also increased, in later phases of development,which suggests a relationship with active enamel production. The findings suggest that the pattern of FGF-10 presence should be analyzed when studying dental morphological anomalies, suchas the palato-gingival groove.
Acknowledgments
The authors would like to thank Antônia Vilma Lopes for her valuable support in the pursuit of this research.
Collaborators
JP ENNES coordinated the research project and participated in all phases of the development of the article. VS LARA was responsible for the standardizing the antibody, checking the slides, photomicrography and the composition of the article. IA TOZETTI supervised the development of the immunohistochemical technique, the checking of the slides and took part in the composition of the article. DM ANTUNES supervised the histotechnique, checking of the slides and took part in the composition of the article. GB BORGATO participated in the development of the immunohistochemical technique of the specimensfrom 0.5 to 3.5 days, the checking of the slides, photomicrography and the composition of the article.
REFERENCES
1. Thesleff I. The genetics basis of tooth development and dental defects. Am J Med Genet A. 2006;140(23):2530-5. doi: 10.1002/ajmg.a.31360. [ Links ]
2. Zhang YD, Chen Z, Song YQ, Liu C, Chen TP. Making a tooth: growth factors, transcription factors and stem cells. Cell Research. 2005;15:301-16. doi: doi:10.1038/sj.cr.7290299.
3. Vastardis H. The genetics of human tooth agenesis: New discoveries for understanding dental anomalies. Am J Orthodont Dentofac Orthop. 2000;117(6): 650-6. doi: 10.1016/S0889- 5406(00)70173-9.
4. Fujiwara N, Kagiya T, Ishizeki K, Harada H. Molecular mechanisms regulating transition from crow to root during formation in the development of mouse root. J Oral Biosci. 2008;50(3):154-9. doi: 10.1016/S1349-0079(08)80002-3.
5. Nakatomi M, Morita K, Eto K, Ota MS. Sonic hedhog signaling is important in tooth root development. J Dent Res. 2006;85(5):427-31. doi: 10.1177/154405910608500506.
6. Seo BM, Miuta M, Gronthos S, Batouli S, Brahim J, Young M, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149-55. doi: 10.1016/S0140-6736(04)16627-0.
7. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Liu H, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal. pone.0000079.
8. Tummers M, Thesleff I. Root or crown: a development choice orchestrated by differential regulation of epithelial stem cells niche in tooth of two rodent species. Development. 2003;130(6):1049-57. doi: 10.1242/dev.00332.
9. Yashimiro T, Tummmers M, Thesleff I. Expression of bone morphogenetic proteins and Msx gene during root formation. J Dent Res. 2003;82(3):172-6. doi: 10.1177/154405910308200305.
10. Lohi M, Tucker AS, Sharp P. Expression of axin2 indicates a role for canonical wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn. 2010;239(1):160-7. doi: 10.1002/dvdy.22047.
11. Li J, Huang X, Xu X, Mayo J, Bringas Jr P, Jiang R, et al. SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development. 2011;138(10):1977-89. doi: 10.1242/dev.061341.
12. Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root and periodontium. Tissue Eng. 2006;12(8):2069-75. doi: 10.1089/ ten.2006.12.2069.
13. Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227-30. doi:10.1038/nmeth1012.
14. Guo W, Gong K, Shi H, Zhu G, He Y, et al. Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials. 2012;33(5):1291-302. doi: 10.1016/j. biomaterials.2011.09.068.
15. Kettunen P, Laurikkala J, Itäranta P, Vainio S, Itoh N, Thesleff I. Association of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev Dyn. 2000;219(3):322- 32. doi: 10.1002/1097-0177(2000)9999:9999<::AIDDVDY1062> 3.0.CO;2-J.
16. Nakatomi M, Wang XP, Key D, Lund JJ, Turbe-Doan A, Kirst A, et al. Genetic interactions between Pax9 and Msx1 regulate lip development and several stages of tooth morphogenesis. Dev Biol. 2010;340(2):438-49. doi: 10.1016/j.ydbio.2010.01.031.
17. Yokohama-Tamaki T, Oshima H, Fujiwara N, Takada Y, Ichimori Y, Wakisaka S, et al. Cessation of Ffg-10 signaling resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133:1359-66.
18. Harada H, Ichimori Y, Yokohma TT, Ohshima H, Kawano S, Katsube K, et al. Stratum intermedium lineage diverges from ameloblast lineage via Notch signaling. Biochem Biophys Res Commun. 2006;340(2):611-6. doi: 10.1016/j.bbrc.2005.12.053.
19. Stemberger LA. Immunocytochemistry. New York: Englewood Cliffs; 1978.
20. Harada H, Kettunen P, Jung H, Mustonen T, Wang A, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biology. 1999;147(1):105-20. doi: 10.1083/jcb.147.1.105.
21. Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, et al. FGF-10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129(6):1533-41.
 Correspondence to:
Correspondence to:
JPENNES
e-mail: jennes@ig.com.br
Received on: 1/2/2012
Final version resubmitted on: 21/6/2012
Approved on: 30/8/2012













