Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.1 Porto Alegre Jan./Mar. 2013
ORIGINAL / ORIGINAL
Characterization of the surfaces of dental implants commercial in scanning electron microscopy / energy dispersive spectroscopy
Caracterização das superfícies de implantes dentais comerciais em microscopia eletrônica de varredura / espectroscopia por energia dispersiva
Ronan Miranda VIEIRAI; Fátima Maria NAMENII; João GALAN JÚNIORIII
I Universidade Veiga de Almeida, Centro de Saúde e Pesquisa
II Universidade Federal Fluminense, Faculdade de Odontologia. Rio de Janeiro, RJ, Brasil
III Universidade Estadual do Rio de Janeiro, Faculdade de Odontologia. Rio de Janeiro, RJ, Brasil
ABSTRACT
Objective
To characterize different implant systems morphologically and chemically.
Methods
Six totally pure titanium implants were standardized for size (length, diameter and platform), as follows: Osseotite® (Biomet 3i, São Paulo, Brazil - surface treated with acid), Timatax® (Neodent®, Curitiba, Brazil - surface treated with grit-blasting and acid), Máster Porous® (Conexão Sistema de Próteses, Arujá, Brazil - surface treated with acid), Externo Laser® (Serson® Implant, São Paulo, Brazil - surface treated with laser), Revolution® (Sin, São Paulo, Brazil - surface treated with acid) and External Hex® (Titanium Fix, São José dos Campos, Brazil - surface treated with grit-blasting). For all makes, two implants were analyzed with surfaces modified via grit-blasting and/or acid-etching and laser bombardment.
Results
The implants Titamax® and External Hex (Titanium Fix, São José dos Campos, Brazil) exhibited characteristics of roughness through the blasting of particles, and OSSEOTITE®, Revolution® Implant (Sin, São Paulo, Brazil) and porous Master® (Connection System Prostheses, Aruja, Brazil) had porous characteristics analyzed by a scanning electron microscope (SEM) and energy dispersive spectroscopy (EDS). The only implant surface that did not change was the External Hex Laser® (Serson® Implant, São Paulo, Brazil). The Kolmogorov-Smirnov identified only one difference at a level of significance of 0.07 (p = 0.07) between the implants External Laser® (Serson® Implant, São Paulo, Brazil) and Timatax® (Neodent®, Curitiba, Brazil) compared to the others studied.
Conclusion
It may be concluded that the analysis of the samples showed an increase in impurities after surface modification, the surface treatment influenced the changes in surface morphology and modifications in the presence of contaminants, there were morphological differences between implants from different manufacturers. The elements found suggest that implants have shortcomings with regard to the final cleaning process before marketing.
Indexing terms: Anatomy & Histology. Dental implants. Scanning Electron Microscopy.
RESUMO
Objetivo
Caracterizar morfológica e quimicamente diferentes sistemas de implantes.
Métodos
Seis implantes de titânio total puro Osseotite® (Biomet 3i - superfície tratada com ácido), Timatax® (Neodent - superfície tratada com jateamento + ácido), Máster Porous® (Conexão Sistema de Próteses - superfície tratada com ácido), Externo Laser® (Serson Implant - superfície tratada com laser), Revolution® (Sin - superfície tratada com ácido) e External Hex® (Titanium Fix - superfície tratada com jateamento) foram padronizados em relação ao seu tamanho (comprimento, diâmetro e plataforma) e, para todas as marcas foram analisados dois implantes com superfícies modificadas por jateamento e/ou condicionamento ácido e com bombardeamento por Laser.
Resultados
Os implantes Titamax® e Implant External Hex® (Titanium Fix, São José dos Campos, Brasil) apresentaram características de rugosidade promovidas por jateamento de partículas, e o Osseotite®, Implant Revolution® (Sin, São Paulo, Brasil) e Máster Porous® (Conexão Sistema de Próteses, Arujá, Brasil) apresentaram características porosas analisados por microscópio eletrônico de varredura (MEV) e espectroscopia por energia dispersiva (EDS) . O único implante que não apresentou alterações superficiais foi o Sextavado Externo Laser® (Serson® Implant, São Paulo, Brasil). O teste de Kolmogorov-Smirnov identificou apenas uma diferença ao nível de 0,07 (p = 0,07) entre os implantes Externo Laser® (Serson® Implant, São Paulo, Brasil) e Timatax® (Neodent®, Curitiba, Brasil) frente aos demais estudados.
Conclusão
Pode-se concluir que na análise das amostras houve um aumento de impurezas após modificações na superfície; o tratamento de superfície influenciou na morfologia da superfície e modificações na presença de contaminantes; houve diferenças morfológicas entre implantes de diferentes fabricantes. Os elementos encontrados sugerem que os implantes apresentam deficiências considerando o processo de limpeza final antes da comercialização.
Termos de indexação: Anatomia & Histologia. Implantes dentários. Microscopia Eletrônica de Varredura.
INTRODUCTION
With the advent of osseointegration, dental implants began to be more widely used, with the primary aim of minimizing the consequences of total or partial edentulism. As a result, many techniques and proposals for carrying out dental implantation were mooted, though many of them were only based on prior experience, which resulted in high failure rates. This led to researchers proposing and describing that the success of an implant is dependent on the interrelationship of various components, including the characteristics of the implant surface itself and contamination by microorganisms which depict virulence factors capable of damaging the peri-implant tissue1.
In living tissue, the dental implant is considered to be a foreign body which interacts in various ways with the environment. This could be an affront to chemical, physiological or mechanical life2.
The metals used for Implants have been selected based on a number of factors: their biomechanical properties; prior processing experience, treatment, machining and finishing and packing for sterilization3.
When exposed to the air, titanium (Ti) immediately forms an oxide layer that attains a thickness of between 2 nm and 10 nm in just one second and provides resistance to corrosion. Due to this high rate of passivity, control of thickness, rapid formation, resistance to chemical attack, catalyzing activity for a number of chemical reactions and compatible elasticity modulus between the titanium oxide and the bone, Ti is the material of choice for endosseous implants3-4.
Commercially pure titanium (cp-Ti) has been the material of choice for the production of endosseous implants as it is a metal which allows a favorable tissue reaction, affords chemical stability to the components, stimulates cell activity in the formation of the bone matrix, has a high resistance to corrosion and does not cause hypersensitive or immunological reactions. The layer of titanium dioxide (TiO2) is responsible for the intimate union, known as osseointegration or functional ankylosis, between the mineralized bone and the implant surface4-10. There are many types of phenomena on the surface that have an impact on the interaction between the titanium and the biological tissue. Surface properties such as wettability, load, microstructural and chemical stability are just some of the parameters that must be considered for interactions where the contact with the blood, water molecules and small ions look for the surface first, followed by proteins of high molecular weight and by cells (platelets and leukocytes) defining interaction with the material; the interactions of the surrounding biological system will be with the material, the inorganic phase, water and ions, narrow and broad absorption of biomolecules or initial cell adhesion3,6,10.
The effect of rough surfaces on the formation of the oxide layer and on cell aggregation has been the subject of several studies. However no alterations were observed in the oxide layer, nor were there any biological implications. Moreover, cell aggregation seems to be more favorable to roughness produced by grit-blasting (Ra = 0.7 to 0.9 μm) when compared to polished surfaces (Ra = 0.04 μm) or "scratched" specimens (Ra = 0.1 to 0.2 μm), being composed primarily of TiO2, with oxygen, carbon and nitrogen as contaminants. Inorganic contaminants, comprising small molecules of sodium, chlorine, silica, calcium, phosphorous and sulfur, are found, but not in all of the samples. Samples with an oxide layer less than 100 nm thick are considered to be amorphous8,11.
During the implantation procedure, predictably, a layer of hydrated oxide grows over the titanium. The interaction between the bone and the metal depends on the metabolic activity in the site of the implantation. Experimental models show that titanium in vitro forms a compound of titanium peroxide expected to interact with the hydrogen peroxide, a type that is linked to inflammatory cells6. However the biological meaning is unclear, but it was suggested that in vivo the interaction between the titanium and the hydrogen peroxide could have an influence on the dissolution of the material in the tissues and an initial inflammatory response due to the envelopment of the tissue around the implant4. The titanium, by means of its surface, participates in the activation of the immune system and also intrinsically activates blood clotting. Moreover, it is highly thrombogenic when there is contact. The bone appears to be separated from the implant surface by a fine layer, 50 nm thick5. This is a consistent finding in studies on animals. Bone can grow on the surface of the implant, suggesting that the influence of implant design, surgical procedures and mechanical load are important3,5. These effects are highly dependent on the surface properties of the materials and of the modification of these properties through handling and biological systems3,5,7.
Different surface treatments are used to change the topography and roughness of the titanium in order to increase osseointegration, usually leading to a better mechanical and biological anchorage. Acid-etching and acid attack are widely employed and may be used in their simple form or after grit-blasting (a process known as SLA) demonstrating an increase in the levels and quantity of bone formation on the surface of the implant8,12.
Bearing in mind that acid-etching causes different levels of corrosion on the implants and modifies their surfaces with the increase in surface roughness, Sykaras et al.3, Conteno et al.12 and Pimenta & Castro13 described a technique that embraces surfaces of pure titanium, depending on the concentration of the acid and the length of exposure. In this way it is possible, using the same chemical treatment, to obtain both large depressions and slight roughness.
As far as Ellingsen14 is concerned, when an implant is placed, a series of reactions occur on the surface. The implant is exposed to a series of different ions, polysaccharides, carbohydrates and proteins and also to cells like chondroblasts, fibroblasts and osteoblasts that react with the surface. The initial reactions between the tissue components and the surface of the implant guide future reactions and determine biological activity and subsequently the cell responses to the surface. The tissue response depends on the nature of the surface and its chemical properties, which influence the nature of the subsequent composition of the protein film which accumulates inside the surface. The author reported that investigations into osteoblast response to the synthesis of hydroxyapatite are indicative of differences in the manufacturing. Analyses using x-ray diffraction showed the presence of calcium Ions and small differences in impurities through carbon, sodium, silicon and aluminum. The fluorine ions indicate an increase in bone activity.
According to Steinemann4, corrosion via holes and also via fissures, is a form of localized degradation of the material and occurs more with alloys of aluminum, stainless steel and titanium. Holes begin to form in imperfections present in the oxide film in the region, due to the concentration of chlorine ions, without the oxygen being displaced. It may also be noted that the holes are nucleated at points which contain traces of iron, it being the case that the presence of this element establishes a difference in electrochemical potential. As a result, titanium alloys for use in implants should not contain or possess traces of iron and, during manufacture and handling of the parts, contamination of the alloy by this element must be avoided.
Wieland10 conducted a review of the techniques used for the topographic characterization of the surface of implants and confirmed that the choice of technique employed is intimately related to the sur face texture or variations, the lateral and vertical dimensions being of importance. They also stressed that, due to distortions, artifacts of technique, damage to the surface and technical limitations, the best methods for analyzing rough surfaces are laser profilometry and stereo scanning electron microscopy, firstly, because these attain the dimensional level of interest and secondly because these techniques do not stimulate contact, therefore they do not destroy the surface; and thirdly because in the light of the morphological complex of the surface, it promotes a high rate of discoveries on the surface that provide properties favorable to bone integration.
The machined implants evaluated by Davies7 exhibited low contamination by hydrocarbons with a significant presence of TiO2, providing strong evidence that the material is covered by a fine layer of natural oxide (growth at ambient temperatures) that is well defined with calculus between 5 and 6 nm thick, and highly reproducible.
Binon15 carried out a review of the literature into the characteristics of implants, including their surfaces, and reported that the bone-implant interface has undergone significant advances. The original surface of the implants is machined titanium5. However, the market offers a variety of surface modifications for implants, such as: TPS® (Straumann, Basel, Switzerland), HA-Coated® (Zimmer, Warsaw, USA), Endopore® (Sybron Implant Solutions, Orange, USA), TiOblast® (Astratech, Mölndal, Sweden), SLA® (Straumann, Basel, Switzerland), Osseotite® (BIOMET 3i, Palm Beach, USA), Osteo® (Osteo Implant Corporation, New Castle, USA), RBM® (ACE, Madrid, Spain), MTX® (Zimmer, Warsaw, USA), amongst others. This variety is due to the specific details that exist in production that differentiate the surfaces.
Hayakawa et al.16 conducted an in vivo study to compare the amount of bone contact in four different types of surfaces that were machined, blasted with abrasives and which used calcium phosphate (CaP) spray. After 12 months, rabbits were sacrificed and the bone-implant interfaces were histomorphometrically and histologically analyzed. Implants with CaP produced a greater bone count on the implants, the conclusion being that coverage with CaP is beneficial, favoring bone response in the healing phase.
Orzini et al.17 carried out a surface analysis of titanium implants that had been machined versus those that had been grit-blasted and acid-etched (SLA), consisting of 10 machined implants and 10 with an SLA surface. Etching with SLA is grit-blasting with Al2O3 particles with sizes from 250 to 500 μm and, after blasting, etching with hydrofluoridric acid and 30% nitric acid to eliminate alumina particles, where average surface roughness was found: control (0.75 μm) grit-blasted and SLA (2.15 μm). The results showed that the surfaces had no cytotoxic effects due to the absence of aluminum.
Placko et al.8 examined the effects of different treatments (polished, electropolished and grit-blasted) on the morphology and chemistry of the surface of commercially pure titanium metal and titanium alloy with 6% titanium, 4% vanadium. The structure and composition of the surfaces were evaluated using scanning electron microscopy (SEM), atomic force microscopy (AFM), energy dispersive spectroscopy (EDS), Auger microprobe analysis and photoelectronic spectroscopy via x-ray. On a broad scale, the surface roughness values were approximately identical for grit-blasted and electropolished samples while, on a smaller scale, electropolished and polished samples showed approximately identical roughness values. The composition of the surface oxide was found, being primarily TiO2 for both materials and for all surface treatments. Vanadium was not seen in the analyses using x-ray photoelectronic spectroscopy or via the Auger microprobe analysis of the alloys, indicating possible surface depletion. Calcium was present in the grit-blasted samples. Calcium and chlorine were detected on the electropolished samples.
Describing the surface topography, Sykaras et al.3 reported that it is related to the degree of surface roughness and to the orientation of the surface irregularities. The different processes result in little topographical difference. Examples of methods used to alter the topography of implant surfaces include electropolishing, stripping, grit-blasting, plasma spraying, superficial adhesion and photolithography. There are four grades of cp-Ti which mostly vary in terms of the quantity of oxygen, the largest being 0.4% and the smallest 0.18%. The properties of the oxide, however, are not affected, but the mechanical properties are altered. Traces of nickel, carbon, hydrogen and iron have been detected. These add stability and improve the mechanical and physical/chemical properties. Iron is added to increase resistance to corrosion and aluminum to increase elasticity and reduce density, while the vanadium acts as an excavator of aluminum to prevent corrosion.
Amarante & Lima18 analyzed the results presented in the literature on the surfaces of implants treated with titanium plasma (TPS), and those blasted with particles and treated with acid (SLA). The surface texture was far and away the most marked feature in the promotion of osseointegration, there being bone deposition on both smooth and rough surfaces. However the latter plays a dominant role in the percentage of bone apposition on the implant surface as well as the speed with which this apposition occurs.
Joly & Lima19 conducted an SEM evaluation on the characteristics of the surfaces of implants coated in ceramic calcium phosphate, with one-stage and two-stage titanium plasma spray, with the aim of evaluating the split that formed between a conical abutment and the implants. They found no statistically significant difference between implants in the length of the split.
Müeller et al.20 checked the amount of bone contact on titanium surfaces without surface treatment, with AL2O3 blasting and with bioceramics. The effects were experimentally investigated on 27 rabbits 7, 28 and 84 days after implantation of the cylinders. The results showed that both AL2O3 and bioceramics produced greater metal-bone contact than the control group and, from day 28, the samples blasted with bioceramics produced more bone in contact than the implants blasted with AL2O3.
Bathomarco et al.21 carried out an analysis of cp- Ti used in dental implants, using atomic force microscopy (AFM), with the aim of evaluating the morphology, roughness and surface area of four samples. One sample was composed of a machined surface and the other three had surface treatment that consisted of etching in baths of hydrochloric acid, followed by hydrofluoridric acid and nitric acid and another sample that was blasted with titanium oxide (TiO2) particles 50 μm in diameter and at a pressure of 30 psi. The last sample received blasting and acid-etching treatment. All the samples were cleaned with acetone in an ultrasonic bath for 15 minutes. Using AFM, images were acquired of the average surface roughness and the area was calculated. They concluded that the implants that received blasting treatment had the roughest surfaces, followed by those which were acid-etched. The grit-blasted implants that received acid treatment showed a reduction in surface roughness and in the control group it was the machined implants. They also observed that the surfaces with increased surface area provided a reduction in the surface angle of contact. From the point of view of surface energy, the implants blasted with TiO2 should have more fine points assessed for osteoblasts, which would help with adherence to hydrophobic surfaces. However, the tradeoff between surface area and wettability seems to have reached maximization as far as osseointegration is concerned.
Elias22 presented a study comparing the morphology of four types of implant surface; they were machined, subjected to acid-etching, blasted and subjected to acid-etching and anodized. The surfaces were of ASTM grade 4 cp-Ti and the acid bath mixture contained HNO3 + HCl; the blasting was carried out using particles of titanium oxide and, when followed by acid treatment, HNO3 was used. As for the morphological analyses, these were carried out using SEM and the topographic analyses using a roughness meter. The results showed that the implants with surfaces treated with acid were more uniform and not as rough. The implants that were blasted and then received acid treatment presented microcavities of different shapes and sizes. Surfaces with electrochemical treatment exhibited protrusions in the form of small cones. They noted a tendency for an increase in the number of cells adhering to the implant surfaces with increased roughness. Among the surfaces analyzed, implants with electrochemical treatment presented surface characteristics and cell behavior that were more favorable to the process of osseointegration.
Wennerberg et al.23 performed an in vivo and in vitro analysis of the correlation that existed between the dissociation of Ti ions with the roughness of the surface of implants with particle-modified surfaces and those with smooth surfaces, at 12 weeks and at one year, using for the analysis a fluorescence x-ray spectroscope (in vivo) and a secondary mass ion spectroscope (in vitro). A slight increase in ions was observed close to the surfaces with greater roughness and a decrease for all surfaces, at a focal length greater than 400 μm, in both periods, and the researchers were able to conclude that there was no increase in the level of dissociation of Ti ions with the variance in surface roughness, either in vivo or in vitro.
Brunski et al.24 reported that surface roughness was related to the increased bone retention in the implants. The authors carried out a review of the differences found between the studies that evaluated the removal torque values for implants with smooth and rough surfaces and they proposed that the results found in the various studies were wrong to conclude there was an increase in torque for the removal of implants with treated surfaces, as it was a mistake not to take into consideration the increase in the surface area of the implant due to the treatment of the bone-implant contact surface, which typified an increase in torque.
Gaetti-Jardim Júnior et al.1 evaluated the surface characteristics of Sin® implants (Sin, São Paulo, Brazil), specially developed for research studies, as well as the existence of residual contamination thereon. A total of six SIN grade 2 cp-Ti alloy mini-implants were used, all of which had their surfaces treated with a double acid attack. The methodology consisted of the use of a UBM® (UBM Corporation, Sunnyvale, USA) interferometer for analyzing three-dimensional surfaces, using the laser interferometry technique. Then, in order to evaluate the presence of residual contaminants, they were removed from their casings inside the laminar flow chamber in order to comply with basic disinfection requirements. They were subsequently transferred to tubes containing brain and heart infusion broth (Difco®, BD, Franklin Lakes, USA), supplemented with yeast extract (0.5%), and to tubes containing tioglycolate broth (Difco®, BD, Franklin Lakes, USA) with calcium carbonate added, and were incubated at 37°C for 72 hours and 14 days, respectively. The presence of microbial growth was assessed by computing the absorbance of the culture medium, with the aid of a spectrophotometer (A380 nm). As a negative control, to determine absorbency, they used their own culture media without microbial growth. Samples were also inoculated on dishes containing Sabouraud-Dextrose agar and incubated in aerobioses at 37°C and at room temperature, for 72 hours and 14 days, respectively, to evaluate the presence of yeast-forming, fibrous fungi on the implants. In the quest for a complete characterization of the surface topography of the implants, numerical data were obtained by means of a quantitative analysis provided by the roughness parameters considered by the aforementioned study: Average Roughness (Sa) = 0.438 m; Average Quadratic Roughness (Sq) = 0.567 m; Skewness (Ssk) = 0.487; Kurtosis (Sku) = 3.68 and Reduced Valley Depth (Svk) = 0.481 m. The values obtained from the evaluation of microbial growth by the spectrophotometer were submitted for statistical analysis using the Kruskal-Wallis test with a level of significance of 5%. Regardless of the presence of microimperfections characteristic to these types of implants or the surface topography they present, the presence of microorganisms was not found on their surface.
Reviewing the literature to ascertain the effects of the topography of implants on integration with soft tissue, Rompen et al.25 concluded that, despite the rough surface having better conditions for the formation and stabilization of the fibrin network for cell adhesion, machined surfaces present, in vivo, better adhesion to the titanium.
Qahash et al.26 analyzed in vitro the behavior of machined implants with a treated surface in relation to the formation of new bone and native bone formed by induction through the morphogenetic protein-2 of recombined human bone. The results showed that there was no difference between bone densities in the two types of implants, however for those implants with treated surfaces, there was a big increase in bone-implant contact. They concluded that the acid-etching of the titanium implants has a positive effect on the osseointegration of new bone and native bone and that differences in bone density do not seem to have an influence on this effect.
Tavares et al.27 performed a comparative study, in vivo, on the influence of chemical treatment of the surface on bone formation, using MK III® implants that received treatment with H2SO4 and H2O2 and others that received no form of treatment. They carried out the placing of the implants in the lower jaws of dogs which were to be subsequently sacrificed between three and eight weeks after the surgical procedure, for an analysis of the percentage of bone-implant contact, the result of which was a significant increase in contact osteogenesis, suggesting that this type of surface could be beneficial for the use of immediate load implants.
This study, therefore, aimed to perform a quantitative analysis of the chemical and morphological properties of the surface of a number of implants marketed by different companies in Brazil, with the intention of rating, morphologically and chemically, the surface of the implants and the possible degree of surface contamination.
METHODS
Six totally pure titanium implants were standardized for size (length, diameter and platform), as follows: Osseotite® (Biomet 3i, São Paulo, Brazil - surface treated with acid), Timatax® (Neodent®, Curitiba, Brazil - surface treated with grit-blasting and acid), Máster Porous® (Conexão Sistema de Próteses, Arujá, Brazil - surface treated with acid), Externo Laser® (Serson® Implant, São Paulo, Brazil - surface treated with laser), Revolution® (Sin, São Paulo, Brazil - surface treated with acid) and External Hex® (Titanium Fix, São José dos Campos, Brazil - surface treated with grit-blasting). For all makes, two implants were analyzed with surfaces modified via grit-blasting and/or acid-etching and laser bombardment.
The implants were standardized to 3.75 x 13 mm, external hexagon with conveyor belt to facilitate handling as well as to avoid possible contamination, except for the Timatax® implant (Neodent®, Curitiba, Brazil) which was conveyed using an internal torque drill, as the company no longer makes implants with fitters and, for all the makes, implants with modified surfaces were analyzed. The implant samples were divided into two groups for the sequence of analyses which contained one implant the sequence of analyses which contained one implant for each make. The variables relating to morphology and chemical composition were observed, with two samples of each implant and in three preselected regions: cervical, mid-third and apical.
For this study, the JEOL JSM - 6460LV microscope was used belonging to the Federal University of Rio de Janeiro, COPPE - Department of Metallurgical Engineering, Electron Microscopy Laboratory. The microscope operated at an acceleration voltage of 20 keV.
To reconstruct the SEM images, an image building software application was used called Jeol Scanning Electron Microscopy. The size of the scanned areas was dependent on the magnification and could be checked against the scale on the photomicrographs. All the images were at a resolution of 1280 x 960 pixels (dots per square inch). Observations were made using magnifications of 30x, 500x and 2000x.
The EDS microprobe was used as it produces a characterization of the biomaterial using a multiple analytical technique which was critical to providing information about the surface of the biomaterials and due to its sensitivity and ability to detect all the elements with an atomic number above two7.
In order to produce the spectra of the implant surfaces, the areas were first selected using the lowest amplification. Due to the need to get closer to use the EDS, the analyses were conducted at magnifications of 500x and 2000x in the same field of view previously chosen and focused for a magnification of 30x. The regions analyzed by the EDS were the same as those analyzed morphologically. The marking of the points was then carried out on the areas that were different from the pattern of the field viewed.
The use of ANOVA was tried, consisting of a parametric procedure used to check if the averages vary across the analyzed groups. The assumptions used to perform this test are: normality (the data should be distributed according to the normal curve); homoscedasticity (same variances across the groups) and the group sizes should be similar. In the data that were verified, normality was not observed via the Shapiro-Wilk tests. However, the breaching of this assumption did not make it impossible to use ANOVA, as homoscedasticity is a more important assumption and this was found in the sample by using the Levene test (a check to see if variances are the same across the tested groups). However, with the use of ANOVA, significance was not found, showing that there was no evidence to refute the hypothesis that one average was statistically different from the rest.
Accordingly, it was attempted to verify any difference that might exist between the groups by using other non-parametric statistical tests. First of all the Kruskal- Wallis test was used to identify if a median in one of the groups differed from the others, and again no significance was found in this test. Immediately afterwards, the Mann- Whitney test was applied to check for differences in the medians between groups, comparing pair by pair and Kolmogorov-Smirnov test to make comparisons every two groups. In the Kolmogorov-Smirnov test, it was only possible to identify one difference at a level of 0.07 (p = 0.07) between the implants from Externo Laser® (Serson® Implant, São Paulo, Brazil) and Timatax® (Neodent®, Curitiba, Brazil) versus the others used in the study.
RESULTS
With regard to the surface morphology with SEM, the presence was observed of machining chips, particles, grooves, changes in the surface of the threads and in coloration, topographic characteristics and other features of various sizes on the surface of some implants, suggesting differences between the manufacturing processes of the implants marketed in Brazil (Figures 1 and 2).
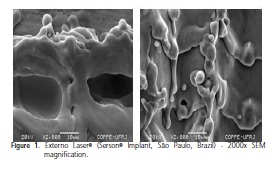
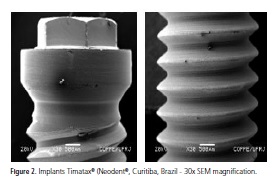
Each implant exhibited its own surface characteristics that are linked to the different manufacturing processes such as: surface treatments and cleaning processes, sterilization and packaging.
Using visual observation of the photomicrographs of the surface, the most notable characteristic of each type of implant was observed: the implants Revolution® (Sin, São Paulo, Brazil), Osseotite® (Biomet 3i, São Paulo, Brazil) and Master Porous® (Conexão Sistema de Próteses, Arujá, Brazil) presented a surface with a porous pattern of an approximate width of between 1 and 10 μm (pore size), which was well standardized and had few grooves, and the Master Porous® implant (Conexão Sistema de Próteses, Arujá, Brazil) had areas without any porosity at all.
The surfaces of the implants Timatax® (Neodent®, Curitiba, Brazil) and External Hex® (Titanium Fix, São José dos Campos, Brazil) possess areas with similar roughness features. One big difference was found with the Externo Laser® implants (Serson® Implant, São Paulo, Brazil) which underwent surface treatment using laser bombardment (according to the manufacturer), and only exhibited pores in the cervical region where we have the beginning of the application of the laser, and the size of these is in excess of 10 μm; particles scattered in the surrounding area were found due to the re-melting caused by the laser, as its surface has a brushed appearance in a lower magnification, and deformed without any defined pore pattern at a higher magnification.
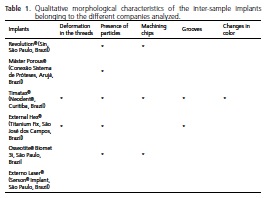
After compiling the results, the following items were presented in Table 1: the qualitative morphological characteristics of the implants, inter-samples of the various manufacturers analyzed, with regard to the morphological alterations on the surface of the dental implants marketed in Brazil.
Amongst the more frequently observed alterations was the presence of both organic and inorganic compounds, which were found in five samples and in all regions, except for the Externo Laser® implants (Serson® Implant, São Paulo, Brazil) which had undergone physical treatment of the surface using laser bombardment. The other analyzed makes underwent surface treatment via the blasting of particles and/or acid-etching which could be the possible cause of the presence of remaining particles.
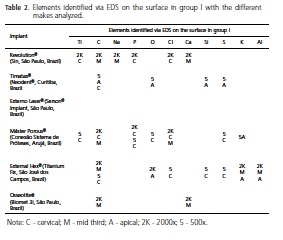
As for the chemical analysis (EDS), following the analysis of 13 points, in eight proposed areas, in two Osseotite® implants (Biomet 3i, São Paulo, Brazil), Ca, C and O were found in the mid third and apical regions of the samples and Cl in the cervical region, however most of the surface is composed of Ti. It should be noted thatin the cervical region, the implant does not show surface treatment as it does in the other regions and, nevertheless, it exhibits contamination by different elements on these different surfaces.
With the Revolution® implants (Sin, São Paulo, Brazil), after analyzing 10 points, in six proposed areas, C, Cl and P were found in the cervical region and in the mid region the elements C, Na, O and Ca were found for all the points analyzed that exhibited contaminating elements, deriving from particles on the surface, except for those identified in the middle of the sample that were inside a surface crater. On the other hand, it is important to stress that the Ti was the most counted element for the proposed analysis.
When 11 points were analyzed, in seven proposed areas, with the Timatax® implants (Neodent®, Curitiba, Brazil), residue was found (cervical and apical) of C (cervical), S, O, Al and Si (apical), but most of the surface was composed of Ti. It is worth pointing out that all the contaminating elements found in the samples were identified in particles that were on the surface. In this case, there is a marked difference amongst the other implants analyzed, as Al was found in the cervical region which received no surface treatment. In the treated region (apex) however, the same was not true, the elements identified in this region being C, O, Si and S.
With the Externo Laser® implants (Serson® Implant, São Paulo, Brazil), eight points were analyzed, in four areas, and no surface contaminant was found, Ti being the only element. Due to the pattern of treatment and the results obtained, evidencing the chemical properties of this surface, a smaller number of points and areas was analyzed as they already demonstrated surface constituents that did not show any visual changes in the pattern.
After analyzing the 11 points, in six proposed areas, of the Máster Porous® implants (Conexão Sistema de Próteses, Arujá, Brazil), particles of Ca, Co, O (cervical) and C, O, Na, Cl and K (apical) were found, however Ti was present in the other regions under observation. However, all the contaminants were observed in group I and group II was seen to be free of contaminants at the marked points.
A total of 13 points were analyzed, in six regions, two from each proposed third of the External Hex® implants (Titanium Fix, São José dos Campos, Brazil), Al and C being found in the mid third of the implant; at the apex, as well as finding C and O and, in the cervical region C, Si, S, Cl and K; however most of the surface contained Ti. One of the characteristics observed in this make of implant was the discovery of Al only in the treated regions and the EDS EDS indicated its presence both on particles and away from them. As for the other elements identified, these were in the cervical region, except for C, in a region in which no surface treatment had been carried out, but which contained particles, and in these particles the presence was found of the other aforementioned elements.
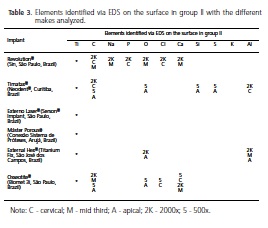
After analysis of the spectra produced by electron dispersion probing, at the marked points, Tables 2 and 3 were produced, showing all the elements identified on the surface of the samples studied in groups I and II, respectively. It was possible to observe that, besides Ti and O, C was the second most frequently occurring element in the samples, followed by P, Cl and Na. S, Si and Al were only identified on two surfaces (Timatax®, (Neodent®, Curitiba, Brazil; External Hex®, Titanium Fix, São José dos Campos, Brazil), and the same occurred with K (Máster Porous® (Conexão Sistema de Próteses, Arujá, Brazil) and Timatax® (Neodent®, Curitiba, Brazil).
DISCUSSION
The era of modern dental implantology began in the 1960s. At that time, in vivo microscope studies that sought to understand how bone tissue initially healed, conducted by Bränemark et al.5 using a titanium cannula, breathed life into the concepts of osseointegration5. Since this time, researchers have been studying the phenomena involved in the interaction between living tissue and titanium impants3,4,6-7. An appropriate tissue response to the biomaterial depends on various factors that involve both implantation as a surgical technique and responses promoted by the receptor5,7. As far as titanium is concerned, one has to consider aspects that relate to its chemical composition as well as the properties inherent in its surface which, ultimately promoted the final conditions for the first events between the biomaterial and the host to result in the success of implant therapy, namely osseointegration5.
Titanium is the material of choice in implant therapy due to its high reactivity. This property accords it an oxide formation at the surface which is extremely stable and compatible when in contact with the air, water or any other electrolyte. This oxide forms spontaneously and almost instantaneously, and is very reproducible3,4,10. As well as being dense and extremely resistant, the oxide protects the surface of the implant from chemical attack (corrosion). So the implant-tissue interface is governed by the layer of oxide and not by the metal itself and it is concluded that, for osseointegration, what matters is the composition of the oxide layer and not the metal4,7.
After choosing a titanium alloy, the factors that will influence the properties, in the oxide layer of the end product to be marketed, such as thickness, chemical composition and microstructure, will depend on pressure, machining velocity, surface treatment, cleaning, sterilization, packaging and storage3-5,7,9,14.
Certain elements have been added to improve the physical/chemical behavior and the alloy's mechanics. However these could lead to contamination of the final layer of oxide on the surface, changing the cell behavior in either a positive or negative way3,11.
This study was developed with the aim of evaluating the possible morphological differences and the presence of potential organic and inorganic contaminants in the six different makes of implant most frequently marketed in Brazil and having modified surfaces, since various authors have demonstrated the importance to osseointegration of the surface characteristics of the titanium oxide layer3-8,11.
At the present time, various analytical methods exist that employ interaction between the surface of the material analyzed and concepts related to quantum physics. The choice of method must cater to the objectives to which this study aspires, namely: a) to analyze the graph of the chemical composition of the surface and determine the possible contamination from processing; b) to evaluate the possible morphological changes on the surfaces as a result of the modifying treatment performed thereon.
The decision to use SEM/EDS was taken as this is the most appropriate tool for morphological evaluation, as well as for the acquisition of data for the chemical characterization of the surfaces analyzed. Through SEM, enlargements were carried out using magnifications of 30x, 500x and 2000x, enabling the identification of the morphological patterns of each surface, as well as the individual characteristics such as: presence of particles, grooves, changes in color, changes in the thread finish, presence of chips3,8 since the different processes result in little topographical differences and this makes it necessary to have at least two dimensions in order to get measurements9. Moreover, without a standard analytical process, it is impossible to compare the values of one study with another23.
Despite the fact that the use of only one method is not sufficient for a complete characterization of a surface, the EDS microprobe is the instrument most recommended for routine microanalysis, particularly in the case of the determination of smaller elements or in situations in which greater spectral resolution is desired; it achieves an analysis of up to 5μm of surface depth.
The six makes of implant used were standardized for size (length, diameter and platform) and for all makes, implants were analyzed with surfaces modified using gritblasting and/or acid-etching as well as laser bombardment. The implants were standardized at 3.75 X 13 mm, external hexagon and for all makes, implants with modified surfaces were analyzed. The variables relating to morphology and chemical composition were observed in two samples (group I and group II) of each implant and on three predetermined areas: cervical, middle third and apical.
There were 2 samples for each implant used in this study; it was not appropriate to use a larger number of samples in this study as there is a difference between each sample with regard to contaminants and morphological characteristics.
The different makes of implant evaluated showed morphological differences in the surfaces and, on an intragroup basis, displayed the same pattern, but as there is insufficient information available in terms of the techniques used to create surface roughness in the marketed samples3, a qualitative analysis was performed and the possible morphological and chemical characteristics found with their surfaces was reported.
As for the morphological characteristics, the present analysis showed that all the surfaces presented some feature or other that made them rough due to specific production details22 and, according to Amarante& Lima18, this plays a dominant role in the percentage of bone apposition on the implant surface as well as the speed with which this apposition occurs24, despite Rompen et al.25 reporting that, in vitro, smooth surfaces produced better cell adhesion. For Qahash et al.26, there are no differences between bone density for smooth and rough surfaces.
According to some authors8,11-12,22, studies with rough surfaces in the formation of the oxide layer and cell aggregation, do not demonstrate any alteration in the latter nor are there any biological implications, and no increase in the levels of Ti dissociation were found23.
A notable feature of the study came in regard to the SERSON implants which received surface treatment with laser bombardment (according to the manufacturer), only presenting pores in the cervical region where we see the start of the application of the laser and these pores were greater than 10 μm; scattered particles in the surrounding area were noted due to the re-melting caused by the laser and its surface has a brushed appearance at a smaller magnification and is deformed without a defined a smaller magnification and is deformed without a defined pore pattern at higher magnification, representative of a surface which is more smooth than rough8,27.
However, in the other implants analyzed, the presence was noted of particles on the surface with the three different magnifications and in all regions, which suggests flaws in the cleaning and sterilization process7. The particles were of different sizes but it was possible to note a greater frequency in the cervical region of the samples where, it is believed, the surface treatments help to eliminate contaminants, being most evident with the Externo Laser® implants (Serson® Implant, São Paulo, Brazil) which received physical treatment, not exhibiting any change in the morphological pattern nor potential surface contaminations through contact with cleaning products.
Alterations in the thread finish as well as grooves were repeatedly seen with the Titamax® implants (Neodent®, Curitiba, Brazil) in the cervical region and middle third and with External Hex® (Titanium Fix, São José dos Campos, Brazil). External Hex® (Titanium Fix, São José dos Campos, Brazil) only in the cervical region for the three magnifications and machining chips were observed in the implants Revolution® (Sin, São Paulo, Brazil), Osseotite® (Biomet 3i, São Paulo, Brazil), Máster Porous® (Conexão Sistema de Próteses, Arujá, Brazil) and Titamax® (Neodent®, Curitiba, Brazil) at the lowest and highest magnifications, in the cervical region. These morphological alterations suggest deficiencies in the process of the machining of the implant that could be due to the machining velocity, the quality of the equipment or even a lack of maintenance of said equipment, demonstrating that the surface treatment procedures help with the cleaning and uniformity of the surface8,11.
An alteration was also observed in the coloration of the surface of the Titamax® implant (Neodent®, Curitiba, Brazil) at the lowest magnification. This finding might indicate a buildup of heat at the time the implant was machined which could impair the characteristics of the titanium oxide, leading to modifications at the interface which could have an adverse impact on osseointegration4-5.
As expected, the surfaces of the cp-Ti implants should only have Ti on the surface. As for the implants, they go through the processes of machining, treatment, cleaning, packaging, sterilization and storage, which could all have an impact on the quality of the component elements of the surface of the implants to be marketed for clinical use3,7-8,11.
With regard to the presence of chemical elements on the surface, it was possible to say that Ti is the primary element present on the analyzed surfaces. Nevertheless, other contaminating elements were identified in the samples, namely: C, Na, P, O, Cl, Ca, Si, S, K and Al.
The presence of oxygen in the samples is due to the instantaneous corrosion of the cp-Ti surfaces, its presence characterizing the formation of TiO2, an oxide which governs the inert chemical behavior of the implants and promotes the ideal biological conditions for the phenomenon of osseointegration and, ultimately, they do not represent contaminating elements3-5,7-8,22.
As for the carbon, this appears as an organic contaminant which could have been acquired in the air, at the time the implants were exposed for the preparation of the samples, or by flaws in the cleaning, sterilization and packaging, as cp-Ti does not have this element in its makeup, and even when it is identified, it appears as particles on the sample surface3,8.
By conducting an inter-brand analysis, it was possible to state that the Externo Laser® implants (Serson® Implant, São Paulo, Brazil) were the only ones not to present surface contaminants, with EDS analysis of the investigated samples, the physical post-treatment of the surface, laser bombardment, as per the manufacturer, confirming that the type of surface treatment interferes with the final chemical pattern of the surface layer of the implants, when compared to the other makes of implant which received treatment via the grit-blasting of the particles and/or acidetching9,11,14. Differences were also found in the spectra of the marked regions amongst implants of other makes.
The External Hex® implants (Titanium Fix, São José dos Campos, Brazil) were the ones exhibiting the highest number of surface contaminants: C, O, Cl, Si, Ca, S, K and Al. There was a difference between the implants in groups I and II in that group I exhibited a low count of C, Si, S, Cl and K in the cervical region and group II, in the same region, did not exhibit any contaminating elements, a region which did not receive surface treatment, suggesting that there may be a flaw in the standardization of the cleaning processes. The presence of these elements tells us that the implants received acid baths in order to clean the surface and that these were not properly removed. By comparing the mid and cervical regions in the two groups, it was found that Al and O were present in both groups and Ca in group II; however, this group showed a high scan count for Al at the apex of the implant. These findings confirm the morphological characteristics of the surface for this make of implant, which exhibits a rough surface with characteristics of blasting and which, given the identification of Al in the treated regions, leads us to believe that the implant underwent blasting treatment with particles of aluminum oxide, as the EDS spectrum was relative to the particles on the surface. This suggests defects in the cleaning process. Another possible reason for the presence of al would be in relation to the constituent elements where we would only be able to identify such an element if the metal used was not cp-Ti but rather an alloy with Al in its composition4. There was a low scan count with regard to calcium and its presence could be a consequence of the cleaning processes due to its precipitation caused by the use of detergents and some form of vehicle contaminated by the calcium, or even by the sterilization8,14.
The analysis of the results obtained for the samples of Titamax® implants (Neodent®, Curitiba, Brazil) showed a varied count of contaminants on their surfaces. The elements found were: C, O, Si, S, Al, Si and S. Aluminum was the only contaminant identified in group II, in the cervical region, all of the others being identified in group I. Si and S were identified in the EDS spectrum in the apical region. This evidence, together with the results of the morphological characteristics of the Titamax® implants (Neodent®, Curitiba, Brazil) suggests that these implants received a surface treatment through blasting with Al and Si based particles, added to the S based acid-etching. The spectra also point to problems with the final cleaning of the implants due to apical contamination and a lack of control over the areas receiving the blasting, since the cervical region is smooth.
The Revolution® implants (Sin, São Paulo, Brazil) also showed a large spectrum of contaminating elements on the surface corresponding to the elements C, Na, O, Ca, P, Cl. In the cervical region, free from any type of surface treatment, the elements C, Cl and P were identified. The smooth morphological feature of this region and the identification of Cl and P suggest that their presence is due to problems with the total removal of acid agents in the final cleaning of the samples analyzed, and the EDS for this identification was carried out on surface particles. In the mid region, however, the other elements Na and Ca were identified, which are also identified in other studies when implants are subjected to autoclave sterilization or when immersed in water14. These characteristics could also indicate deficiencies in the quality of the detergent fluids used to rinse the implants. These could precipitate these elements in an insoluble form. Another possibility might be the anodization processes on these surfaces. In this instance it would be possible to find a more frequent presence of Ca and P particles, but this was not the case22. The spectrum produced using EDS analyses for the Máster Porous® implants (Conexão Sistema de Próteses, Arujá, Brazil) exhibited a large variety of contaminating elements: C, O, Cl, Na, K, Ca and S. In group II, only C was identified as a contaminant. As for group I, the points that displayed elements other than Ti, included areas where the EDS was applied to particles present in the field. This represents a deficiency in the cleaning processes and a lack of quality in the rinsing fluids, sterilization or even the implant's immersion in water14; another possible cause might be the anodization processes on these surfaces, but in this case, we should be able to see more often the presence of Ca. There is a difference between the type of contaminant in relation to the region analyzed, since where the elements found in the treated regions (apex: Na, Cl, K) differ from those found in the region that did not undergo surface treatment (cervical: Ca), the C found in both regions could have been acquired through contact with the air on opening the implants or be organic contamination from the processing3.
Lastly, the Osseotite® implants (Biomet 3i, São Paulo, Brazil) underwent analysis with an EDS probe as with the samples in the previous makes and the following elements were identified: Cl, C, Ca and P. It was found that in group I the only contaminant besides C was Ca, in the middle third of the implant. In group II, the identification of a spectrum for Cl was made in the cervical region. The presence of this element on a smooth surface characterizes deficiencies with the cleaning of the sample in this group. In the other regions, middle third and apex, which received surface treatment, the following elements were identified: Ca, C, P and CL in particulate form.
The chemical composition will promote different reactions in the surrounding medium. The chemical configuration of the surface presents differences in bulk in the material predicted for the methods of preparation and for impurities trapped at the surface9,11.
The presence of at least one of the elements Na, Ca or K on the surface of the studied samples opens a window to further research into the industrial processing conditions for implants, and their true influence on the surface chemistry.
CONCLUSION
Based on the results obtained using SEM and EDS, the following conclusions may be drawn: in the analyzed samples, there was a presence of impurities after modifications to the surface; the surface treatment had an impact on the surface morphology and the presence of contaminants; there were morphological differences amongst the different makes of implant; all the implants
analyzed exhibited particles on their surfaces, with the exception of Externo Laser® (Serson® Implant, São Paulo, Brazil) which only had Ti; in the analyzed samples, the presence of titanium was always found; the Externo Laser® implants (Serson® Implant, São Paulo, Brazil) had a surface morphology consistent with a smooth surface; the Titamax® implants showed the highest spectrum of contaminating elements; carbon and chlorine were the elements most frequently found on the various surfaces; aluminum was only identified on the surface of the External Hex® implants (Titanium Fix, São José dos Campos, Brazil) and Titamax® implants (Neodent®, Curitiba, Brazil); the Osseotite® implant (Biomet 3i, São Paulo, Brazil) exhibited contaminants.
Collaborators
RM VIEIRA was responsible for the collection of samples, identification of implants and their characterization by SEM / EDS and writing of the article. FM NAMEN was responsible for the literature review and writing of the article. J GALAN JUNIOR supervised research and participated in the statistical analysis and writing of the article.
REFERENCES
1. Gaetti-Jardim Junior E, Gaetti-Jardim EC, Oliveira AC, Semenoff Segundo A, Oliveira SR. Características da topografia superficial e de contaminação microbiana de implantes Sin® desenvolvidos para pesquisa. Innov Implant J. 2006;1(2):25-9. [ Links ]
2. Steinemann SG .The insult of foreign body. Eur Cells Mater. 2001; 1(1):1. [ Links ]
3. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000;15(5):675-90. [ Links ]
4. Steinemann SG. Titanium: the material of choice? Periodontol 2000. 1998;17: 7-21.3 [ Links ]
5. Bränemark PI. Surface properties and osseointegration. In: Bränemark PI, Chien S, Grondahl HG, Robinson K. The osseointegration book: from calvarium to calcaneus. Berlin: Quitessence; 2005. [ Links ]
6. Brunski J, Puleo D, Naci A. Biomaterials and biomechanics of oral and maxillofacial implants: current status and future developments. Int J Oral Maxillofac Implants. 2000;15(1):15-46. [ Links ]
7. IDavies JE, Vacanti JP. Bone engineering. Toronto: Squared Inc., 1999. [ Links ]
8. Placko HE, Mishra S, Weimer JJ, Lucas LC. Surface characterization of titanium-based implant materials. Int J Oral Max Implant. 2000;15(3):355-63. [ Links ]
9. Wennerberg A, Albrektsson T. Suggested guidelines for topographic evaluation of Implant Surface. Int J Oral Maxillofac Implants. 2000;15(3):331-4. [ Links ]
10. Wieland M. Measurement and evaluation of the chemical composition and topography of titanium implant surface. In: Davies JE, Vacanti JP. Bone engineering. Toronto: Squared Inc.; 1999. p. 165-82. [ Links ]
11. Maetzu MA, Alava JI, Gay-Escoda C. Ion implantation: surface treatment for improving the bone integration of titanium and Ti6Al4V dental implants. Clin Oral Impl Res. 2003;14(1):57-60. doi: 10.1034/j.1600-0501.2003.140108.x. [ Links ]
12. Conterno G, Pazos L, Parodi MB, Egidi DA, Corengia P. Surface treatment on biomaterials: acid etching on titanium surfaces [abstract] [19th European Conference on Biomaterials; 2005 Sep 11-15; Sorrento, Italy] [ Links ].
13. Pimenta J, Castro F. Chemical modification of pure titanium surfaces for oral implant. Implant Dent. 1990;8(1):86-9. [ Links ]
14. Ellingsen JE. Surface configuration of dental implants. Periodontol 2000. 1998;17(1):36-46. doi: 10.1111/j.1600-0757.1998.tb00121.x. [ Links ]
15. Binon PP. Implants and components: entering the new millennium. Int J Oral Maxillofac Implants. 2000;15(1):76-94. [ Links ]
16. Hayakawa T, Yoshinari M, Nemoto K, Wolke JG, Jansen JA. Effect of surface roughness and calcium phosphate coating on the implant/bone response. Clin Oral Implants Res. 2000;11(4):296-304. doi: 10.1034/j.1600-0501.2000.011004296.x. [ Links ]
17. Orsini G, Assenza B, Scarano A, Piattelli M, Piattelli A. Surface analysis of machined versus sandblasted and acid-etched titanium Implants. Int J Oral Maxillofac Implants. 2000;15(6):779-84. [ Links ]
18. Amarante ES, Lima LA. Otimização das superfícies dos implantes: plasma de titânio e jateamento com areia condicionado por ácido - estado atual. Pesqui Odontol Bras. 2001;15(2):166-73. doi: 10.1590/S1517-74912001000200015. [ Links ]
19. Joly JC, Lima AFM. Characteristics of implant surface and implant abutment gap in two-and one-stage systems. J Appl Oral Sci. 2003;11(2):107-13. doi: 10.1590/S1678-77572003000200005. [ Links ]
20. Müeller WD, Gross U, Fritz T, Voigt C, Fischer P, Berger G, et al. Evaluation of the interface between bone and titanium surfaces being blasted by aluminium oxide or bioceramic particles Clin Oral Implants Res. 2003;14(3):349-56. doi: 10.1034/j.1600-0501.2003.00791.x. [ Links ]
21. Bathomarco RV, Solorzanoa G, Eliasb CN, Priolic R. Atomic force microscopy analysis of different surface treatments of Ti dental implant surfaces. Appl Surf Sci. 2004;233(1-4):29-34. doi: 10.1016/j.apsusc.2004.04.007. [ Links ]
22. Elias CN. Diferentes superfícies dos implantes dentários [resumo] [III Congresso Latino Americano de Órgãos Artificiais e Biomateriais; 2004; Campinas] [ Links ].
23. Wennerberg A, Ide-Ektessabi A, Hatkamata S, Sawase T, Johansson C, Albrektsson T, et al. Titanium release from implants prepared with different surface roughness: an in vitro and in vivo study. Clin Oral Implants Res. 2004;15(5):505-12. doi: 10.1111/j.1600-0501.2004.01053.x. [ Links ]
24. Brunski JB, Glantz PO, Helms JA, Nanci A. Transfer of mechanical load across the interface. In: Bränemark PI, Chien S, Grondahl HG, Robinson K. The osseointegration book: from calvarium to calcaneus. Berlin: Quitessence; 2005. p. 209-49. [ Links ]
25. Rompen E, Domken O, Degidi M, Pontes AE, Piattelli A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration:
a literature review. Clin Oral Impl Res. 2006;17(2):55-67. doi: 10.1111/j.1600-0501.2006.01367.x.
26. Qahash M, Hardwick WR, Rohrer MD, Wozney JM, Wikesjö UM. Surface-etching enhances titanium implant osseointegration in Newly formed (rhBMP-2-Induced) and native bone. Int J Oral Maxillofac Implants. 2007;22(3):472-77. [ Links ]
27. Tavares MG, de Oliveira PT, Nanci A, Hawthorne AC, Rosa AL, Xavier SP. Treatment of a commercial, machined surface titanium implant with H2SO4/H2O2 enhances contact osteogenesis. Clin Oral Impl Res. 2007;18(4):452-8. doi: 10.1111/j.1600-0501.2007.01344.x. [ Links ]
 Correspondence to:
Correspondence to:
RM VIEIRA
Rua Jequitibá, 89, Horto, 35160-306, Ipatinga, MG, Brasil
e-mail: ronanmvieira@gmail.com
Received on: 22/3/2010
Final version resubmitted on: 27/10/2010
Approved on: 11/2/2011













