Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.1 Porto Alegre Jan./Mar. 2013
ORIGINAL / ORIGINAL
Sterilization in the dental private sector
Esterilização no serviço odontológico privado
Ana Paula de NARDOI; Tatiana Gonçalves ROMANOI; Ana Giselle Aguiar DIASI; Gustav GUIMARÃESI
I Faculdade São Lucas, Curso de Odontologia
ABSTRACT
Objective
To evaluate the quality of the sterilization process in private dental clinics in Porto Velho, Rondônia.
Methods
A sample study was conducted with 100 dental clinics, by way of a questionnaire about procedures related to the sterilization process and implementation of biological monitoring of the equipment, using spores of Bacillus subtilis for the oven and Geobacillus stearothermophilus for the autoclaves.
Results
There was a predominant use of autoclaves for the sterilization of dental materials (72%); Among the equipment evaluated, 7(25%) ovens tested positive (ineffective sterilization) and no autoclaves (0%) produced positive results, demonstrating the effectiveness of the sterilization process. The following parameters, necessary for quality assurance of the sterilization process were found to be inadequate: incorrect time/temperature ratios (100% for autoclaves and 84.6% for ovens); Lack of thermometers on ovens (28%), absence of biological monitoring for control of sterilization (37.3% for ovens and 45.3% for autoclaves); and disinfection of instruments with glutaraldehyde is performed incorrectly. Most dental clinics do not interrupt the cycle of sterilization in ovens and make use of surgical grade sterilization of instruments in autoclaves. The sterilized material is stored in a suitable place.
Conclusion
ITherefore, given the results presented, it may be concluded that most of the private dental clinics in the city of Porto Velho, Rondônia, use autoclave sterilization as the preferred method and that it was effective given the biological indicators used, but the knowledge of dental surgeons regarding the process of sterilization and disinfection was insufficient, which alerts to the need for greater awareness by the professionals. It is hoped that the results can support both education and the monitoring of safe practices for the sterilization of dental instruments in private clinics in Porto Velho, helping and encouraging the academic community with the importance of this issue in training.
Indexing terms: Biological indicators. Exposure to biological agents. Sterilization.
RESUMO
Objetivo
Avaliar a qualidade do processo de esterilização em consultórios odontológicos da rede privada do município de Porto Velho, Rondônia.
Métodos
Realizou-se um estudo de amostra aleatória com 100 consultórios odontológicos, por meio de questionário sobre procedimentos referentes ao processo de esterilização e realização do monitoramento biológico dos equipamentos, utilizando esporos de Bacillus subtilis para a estufa e Geobacillus stearothermophilus para a autoclave.
Resultados
Houve predominantemente o uso da autoclave para esterilização dos materiais odontológicos (72%); Dentre os aparelhos avaliados, 7 (25%) estufas apresentaram resultado positivo (esterilização não efetiva), e nenhuma autoclave (0%) apresentou resultado positivo, demonstrando eficácia no processo de esterilização; encontrou-se inadequação dos seguintes parâmetros necessários à garantia da qualidade do processo de esterilização: relação tempo/temperatura incorretos (100% para autoclaves, 84,6% para estufas); falta de termômetros nas estufas (28%); ausência de monitoramento biológico (37,3% para estufas e 45,3% para autoclaves); e desinfecção dos instrumentais com glutaraldeído é utilizada de maneira incorreta; a maioria dos consultórios odontológicos não interrompe o ciclo de esterilização nas estufas; fazem uso de grau cirúrgico para esterilização dos instrumentos em autoclave; o material esterilizado é armazenado em local adequado.
Conclusão
Portanto diante dos resultados pode-se concluir que, a maioria dos consultórios odontológicos da rede particular do município de Porto Velho, Rondônia, utiliza a autoclave como método de esterilização e que este mostrou ser eficiente diante dos indicadores biológicos utilizados, porém o conhecimento dos cirurgiões-dentistas quanto ao processo de esterilização e desinfecção foi insuficiente, o que vem alertar para a necessidade de uma maior conscientização dos profissionais.
Termos de indexação: Indicadores biológicos. Exposição a agentes biológicos. Esterilização.
INTRODUCTION
The maintenance of the aseptic chain is a biosafety standard that must be followed to the letter in order to avoid the risk of cross-contamination, it being an ethical, moral and legal obligation of each and every dental surgeon1. The dental surgeon comes into intimate contact with the patient and, accordingly, he must maintain strict standards of conduct with regard to infection control within his dental clinic, thereby preserving his own life, that of his patients, the auxiliary staff and their families.
Dental environments possess areas where work activities present physical, chemical and biological risks, both to patients and the professionals working there. Biological risks include contamination by microorganisms on equipment, instruments and materials used in clinical practice, which make cross-infection and occupational infection possible if not properly prepared for use2. Sterilization is the procedure responsible for the complete destruction of all forms of microbial life forms, including resistant varieties such as bacterial spores, micro bacteria, viruses without envelopes or lipids, and also fungi3. Sterilization in hot-air ovens and autoclaves are the physical methods most frequently used in dental clinics.
The efficacy of the sterilization process can be proved via physical, chemical or biological montoring3. Given the infinite number of diseases which professionals or patients could possibly acquire, failing to use chemical and biological indicators must be seen as an act of irresponsibility by the professional, whether in the public service or in private clinics, putting at risk their health and that of the patient.
In dentistry, biological monitoring should be employed at least weekly, and always before the first load of the day and at the end of all maintenance carried out, whether preventive or corrective1.
In a recent study carried out on the efficacy of the Pasteur oven used as sterilizing equipment in dental surgeries in the Central District of Goiania, in the Brazilian state of Goiás, it was demonstrated that biological monitoring of ovens pointed out defects in almost half of the equipment evaluated (45.5%), in which the abnormalities found are explained by the disposal of packaging in the oven, making it difficult for the heat to flow freely; the failure to use an accessory thermometer to check the internal temperature of the equipment; not observing the recommended time/temperature ratio, amongst a number of other factors4. In an analysis conducted by Gonini Junior et al.5 of the application of basic standards of sterilization, disinfectionand gown wear used routinely by dental surgeons and their assistants, it was observed that 10.7% had ovens without external thermometers, 9% never checked the temperature during cycle execution and 15.4% did not recall when it was last done. Prado & Santos, in 20021, evaluating the sterilization conditions of dental materials in the city of Taubaté, in the state of São Paulo, found that many professionals were not sufficiently knowledgeable of sterilization techniques or they were unaware of how the equipment worked.
Bearing in mind the importance of biosafety conduct, the aim of the present study was to ascertain the methods and efficacy of sterilization processes via physical indicators, in private dental clinics in the city of Porto Velho, in the Brazilian state of Rondônia.
METHODS
This study gained the approval of the Ethics in Research Committee, filed as case no. 135/07. The sample was randomly conducted on 100 private dental clinics in the municipality of Porto Velho, in the Brazilian state of Rondônia. The sterilization process was carried out by the dental surgeon's assistants, who were informed about the study's objectives and methodology, signing a free and informed consent form and responding to a questionnaire based on Zardetto et al.6, in respect of the sterilization procedures carried out in their routine dental work.
For an evaluation of the quality of the physical sterilization method, biological indicators were used: Bacillus subtillis (ATCC 6633) for ovens and Geobacillus stearothermophilus (AMSCO 124 BGL) for autoclaves, fabricated in the microbiology laboratory at the University of Taubaté, in the state of Sao Paulo, which was handed to the dental surgeons and/or assistants and placed in metal cases or in packages with the instruments to be sterilized in the oven or autoclave. After sterilization, the envelopes were removed from the oven and/or autoclave with sterilized tweezers (Quinelato Rio Claro-SP, Brazil) and placed in 90 x 15 plastic Petri dishes, previously decontaminated with a solution of chlorhexidine at 2% (FGM do Brasil, Joinville, Brazil) and sealed using adhesive tape (3M do Brasil, Sumaré, Brazil).
The envelopes were taken to the microbiology laboratory at the São Lucas Faculty in Rondônia (FSL), opened aseptically in a laminar flow chamber (Veco CFLH, Campinas, Brazil), and with the aid of sterilized Adson tweezers (Quinelato Rio Claro, Brazil), each filter containing a biological indicator was inserted into a test tube (18 x 180 mm, Brand), containing 15 ml of brain heart infusion broth (BHI, Brain Heart Infusion, Himedia, India). The indicators were incubated at 37ºC for 48 hours, with readings taken every 24 hours.
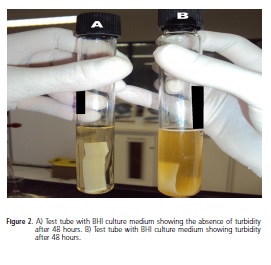
When turbidity was observed, a smear was made and stained using the Gram method to check for the presence of Gram-positive bacilli. If the presence of Grampositive bacilli was confirmed, this culture was left in the oven at 37ºC for at least three more days and then stained smears were made up using the Wirtz-Conklin method for confirmation of spore presence. If the presence of spores was found, the test was considered to be positive.
RESULTS
A total of 180 dental surgeons in charge of dental clinics were invited to participate in the research study, but only 100 agreed to fill out the questionnaires and carry out the analysis of the sterilization process used. Of the 100 samples obtained, it was found that 25 professionals (25%) had returned the questionnaires totally blank. Of the 100 analyzed, 72 carried out a process of sterilization (72%) using autoclaves and 28 using ovens (28%), and 7 clinics used a combination of oven and autoclave (7%).
By attempting to relate time and temperature maintained by the dental surgeons in the sterilization process in autoclaves, it was found that only 55 clinics filled out these data and that the temperature and time used were greater than was necessary, at a temperature of 121ºC and a duration greater than or equal to between 15 and 30 minutes.
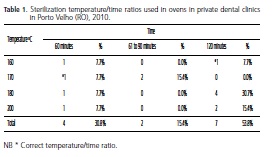
Correlating the temperature vs. time maintained by the dental surgeons in the sterilization process with ovens, Table 1 shows the results found, identifying that the ovens are not being correctly used and just 15.4% of clinics maintain an adequate temperature/time ratio, the most frequent error observed being the use of excessive temperatures.
Of the 28 clinics using ovens, only 13 responded to the question concerning the interruption of the sterilization cycle, and of these 48.36% did not check any response, 28.57% reported that they did not usually place materials inside the oven while other materials were being sterilized and 23.07% reported interrupting the sterilization process.
As for the use of the thermometer in the oven to ascertain temperature, 22.7% replied they did use one, 28% did not and 49.3% did not respond. A thermostat was found to be routinely used in 13.3% of clinics, while 50.7% did not respond, 13.3% stated that they havenever used them, 18.7% sometimes use them and 4% do not use them.
A total of 16% of clinics normally use a sterilization indicator for ovens, while 37.3% reported not using one and 46.7% did not respond. As for the autoclave, 44% stated they used one, 45.3% did not and 10.7% did not respond. As for the indicator used, 24% said adhesive tape, 12% reported using a biological indicator, 9.3% checked "others" (but did not specify which) and 54.7% did not respond.
As for the packaging of instruments to be sterilized, it was found that 50% of dental surgeons who make use of ovens did not respond; 14.28% use a metal case and 35.72% do not package the instruments before placing them in the oven. As for the autoclaves, 54.7% use surgical grade, 9.3% do not package the instruments, 25.3% checked the "others" option and 10.7% did not respond.
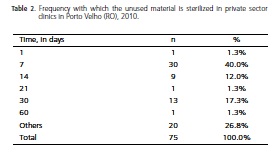
When a sterilized material was not used, the timeframes reported for the execution of a new sterilization procedure can be found in Table 2.
It should be emphasized that the majority of professionals, when filling out the questionnaires, did not specify the duration of disinfection of the clinical instruments.
There was little variation in terms of the places where the sterilized materials were stored, the majority keeping them in a cupboard (64%), while 14.7% kept them in the oven itself, 20% checked the "others" option and 1.3% did not respond.
With regard to biological monitoring in autoclaves, the results were 100% negative, i.e. after being processed in this equipment, all spores became unviable, demonstrating the effectiveness of the sterilization process.
Biological monitoring of the ovens produced positive results in 25% of cases, showing that the sterilization process is not effective, although little used by dental surgeons.

DISCUSSION
Of the 100 samples obtained, it was found that only 75 of the professionals filled out the questionnaires, and of these, 25% of the questions were left blank. Of the 100 clinics visited, 72 had an autoclave (72%), 28 had ovens (28%), and 7 had a combination of both, results similar to those of Corrêa et al.7, who identified that, in the cities of São Manoel and Botucatu, both in the state of São Paulo, the method most frequently used by dental surgeons to sterilize clinical instruments was the autoclave (72.55%), while the other surgeons used ovens (27.45%). Following the same yardstick, Tavares and co-researchers5 found that 38.6% of clinics located in the Central Health District in the municipality of Goiânia, in the state of Goiás, used ovens to sterilize their articles even though they had autoclaves.
Of those dental surgeons that had both an autoclave and oven (n=7), the results were seen to approximate those of Prado & Santos1, who reported that the professionals only use one of the machines in order to economize. According to Vier et al.8, dry heat (dental oven or Pasteur oven) is an effective sterilization method, frequently used in clinical practice, despite the advent and growing use of the autoclave.
As for the time/temperature ratio, a large variation was found with just 5.45% of dental surgeons who used the autoclave placing their instruments in adequate temperature/time combinations, i.e. 121ºC for 15-30 minutes. In the case of ovens, they draw attention to the incorrect answers given, such as: 200ºC for 1 or 2 hours, 180ºC for 1 or 2 hours.
Prado & Santos1, in their evaluation of sterilization conditions of dental materials in clinics in the city of Taubaté, found that 60% of professionals did not know the correct temperature and time for oven sterilization, and highlighted a number of incorrect responses that attracted their attention: "2700C for 2 hours, 1600C for 1 hour, 1500C for 2 hours and 1700C for 3 hours". The Ministry of Health9, via its Health Surveillance permit, establishes sterilization times, namely 1 hour at 170ºC or 2 hours at 160°C. The recommendation of 160ºC for 2 hours is based on the fact that, despite the greater amount of time, at this temperature the materials and instruments are less affected when subjected to sterilization10.
Keeping the oven door closed during the cycle is another factor to note, at the time of sterilization5. Of the professionals that participated in the study, and who used the oven, 28.57% did not normally interrupt the sterilization cycle, 23.07% place instruments into the oven after the cycle has started and 48.36% did not respond to this question. Tavares et al.5 found, in their study, that 87.1% of clinics do not interrupt the sterilization cycle and in 12.9% of clinics, there is intermittent opening of the oven, to add or remove instruments. The author also states that when this occurs, the whole sterilization process is compromised as the article removed did not have sufficient exposure time at the advocated temperature, and when adding a tool, there is a sharp drop in temperature. If this occurs, the elapsed time should be ignored and the cycle repeated6.
As the number of professionals, who did not respond to the question relating to the use of a thermometer, was high, it does not permit us to make comparisons; 22.7% of dental clinics use them and 28% do not. Following the same yardstick, Tavares and coresearchers5, when evaluating the efficacy of the Pasteur oven used as sterilizing equipment in dental clinics, observed that of the 101 clinics evaluated, 65 (64.4%) did not use an accessory thermometer to monitor the oven and only 36 (35.6%) did so, with monitoring taking place between one and three times per cycle.
The correct packing of sterilized instruments is as important as the very process of sterilization since inadequate packing can cause a break in the "chain of sterility"1.
Of the dental surgeons interviewed, 35.72% of those using ovens do not pack the instruments for sterilization. It is important to stress that proper storage guarantees sterility for 15 days1. In the autoclaves, 9.3% did not pack the instruments. It should be emphasized that when storage or handling is not adequately performed, it could contribute to the breaking of the aseptic barrier, leading to the contamination of the material11.
As for the frequency with which unused material is sterilized, a variance was observed. Some clinics reported a period of more than 21 days, however several factors are involved with the question of the validity of sterilized material. The handling of the envelopes was an important variable in the recontamination of materials by bacteria, as the hands are the main transmission path for these microorganisms11.
In 1999, Zardetto et al.6 carried out a study on methods of disinfection and sterilization of clinical instruments and concluded that there was a lack of knowledge in 31.2% of those interviewed about the ideal length of time and temperature for sterilization and they also highlighted the incorrect performance of disinfection or sterilization of semi-critical articles. This was observed in the present study, in which 36% of private clinics use glutaraldehyde at 2% for prior disinfection and only 6.7% use the correct immersion times when disinfecting instruments. However, manufacturers of glutaraldehyde recommend that for chemical disinfection it is necessary for the article to be submerged for at least thirty minutes in a plastic container with lid. In the Brazilian state of São Paulo, the use of glutaraldehyde has been prevented due to its toxicity and risk to the environment. For prior disinfection, glutaraldehyde has been replaced by chlorhexidine at 2% or enzymatic detergent as a result of the imposition in Resolution SS-27 of February 28, 2007, issued by the National Health Surveillance Agency (ANVISA)12. In the state of Rondônia, there is no resolution to prevent its use and therefore this product is still being widely employed.
As for the storage areas for sterilized materials, there were very few variations, with 64% being kept in a cupboard, 14.7% in the oven, 20% chose the "others" option and 1.3% did not respond. Ferreira et al.13 stated that when storing instruments after sterilization, the ideal is to keep them in an exclusive location, in places that are protected from dust, humidity, insects, preferably at least 30cm from the floor, 50cm from the ceiling and 5cm from the walls.
Given the infinite number of diseases that professionals or patients could acquire, not using chemical and biological indicators should be seen as an act of irresponsibility on the part of the professional, in both the public health service and in private practices, putting his/her health and that of the patient at risk.
Corrêa et al.7 conducted a study on the efficacy of sterilization in autoclaves and ovens by means of the use of chemical and biological indicators and concluded that sterilization failure levels range from 8.3% in autoclaves to 21.5% in ovens.
The effectiveness of the sterilization processes carried out in autoclaves could be confirmed by the present study, as all the biological monitoring results proved negative. The results of the biological monitoring with the ovens pointed to a failure rate of 25% (7). Prado & Santos1, evaluating conditions of sterilization in the city of Taubaté, found that of the 50 items of sterilization equipment evaluated, the biological test proved positive for 6 ovens (12%), i.e. the sterilization was not effective while none of the autoclaves (0%) presented positive results.
As far as the question of efficiency and quality of sterilization using the physical method is concerned, autoclaves come out on top in comparison with ovens4. However, we should remember that if all the biosafety rules were observed and the appliances were in perfect working order, sterilization would always be effective1.
For the process of sterilization not to present defects whether it is with ovens or with autoclaves, monitoring should be carried out by evaluating the physical, chemical and biological parameters so that these defects can be corrected before the materials reach the patient14.
The results of the present work demonstrate that the use of autoclaves has increased, being the safest and most effective means of sterilizing materials, with the aim of preventing cross-infection in dental clinics.
CONCLUSION
According to the results found, it was concluded that the autoclave was predominantly used for sterilizing dental materials (72%), and was seen to be the more effective. Biological monitoring of the ovens produced positive results in 7 (25%) appliances, indicating that the sterilization process is not effective, although little used by dental surgeons. Moreover, the following parameters, required to guarantee the quality of the sterilization process, were found to be inadequate: incorrect time/temperature ratios (100% for autoclaves, 84.6% for ovens), lack of thermometers on the ovens (28%), absence of biological monitoring for the control of sterilization (37.3% for ovens and 45.3% for autoclaves); incorrect disinfection of instruments using glutaraldehyde.
It was also concluded that the majority of the dental clinics do not interrupt the oven sterilization cycles and make use of surgical grade to sterilize their instruments in autoclaves, the sterilized material is stored in an adequate location, therefore, given the results presented, it may be concluded that the majority of the private dental clinics in the municipality of Porto Velho, Rondônia, use the autoclave as the preferred method of sterilization and that this was seen to be efficient given the biological indicators used, however the dental surgeons' understanding of the sterilization and disinfection process was insufficient, which alerts us to the need to make professionals more aware. It is expected that the results found can provide support for actions of education and monitoring towards a safe practice of sterilization of dental instruments in the private sector in Porto Velho, Rondônia, contributing to and stimulating the academic classes about the importance of this topic in the professional training.
Collaborators
AP NARDO and TG ROMANO took part in the data collection, the laboratory stage and the composition of the article. AGA DIAS took part in the correction of the article, sending the project to the Ethics Committee, the donation of tests and the composition of the article. G GUIMARÃES participated in the drafting and composition of the article.
REFERENCES
1. Prado MEM, Santos SSF. Avaliação das condições de esterilização de materiais odontológicos, em consultórios na cidade de Taubaté. Rev Bioc Taubaté. 2002;8(1):61-70. [ Links ]
2. Frazão P, Bortolotti MGLB. Desigualdades nas condições de controle de infecção em consultórios odontológicos em município brasileiro. Cad Saúde Pública. 2006;22(5):965-74. [ Links ]
3. Estrela C, Estrela CRS. Esterilização e desinfecção. In: Estrela C, Estrela CRA. Controle de infecção em odontologia. São Paulo: Artes Médicas; 2003. p. 111-25. [ Links ]
4. Tavares SSF, Sousa JT, Tipple AFV, Souza ACS, Pimenta FC, Anders PS. Eficácia da estufa de pasteur como equipamento esterilizante em consultórios odontológicos. Rev Esc Enferm USP.
2008;42(1):160-7. doi: 10.1590/S0080-62342008000100021.
5. Gonini Júnior A, Gonini CAJ, Inada DY, Almeida LG. Nível de aplicação de normas básicas para esterilização, desinfecção e paramentação odontológica. Cient Ciênc Biol Saúde. 2001;3(1):61-8. [ Links ]
6. Zardetto CGDC, Guaré RO, Ciamponi AL. Biossegurança: conhecimento do Cirurgião-dentista sobre esterilização do instrumental clínico. Rev Pós-Grad. 1999;6(3):238-44. [ Links ]
7. Corrêa EG, Castilho ARF, Pereira CVP. Indicadores químicos e biológicos da eficácia de esterilização por autoclave ou estufa. Rev Odontol Ciênc. 2009;24(2):156-60. [ Links ]
8. Vier FV, Lopes AS, Sommer K, Oliveira EPM, Limongi O. Monitoramento da temperatura de estufas odontológicas empregadas para a esterilização do instrumental. Odontol Clín Cient. 2003;2(2):103-8. [ Links ]
9. Brasil. Ministério da Saúde. Secretária de Políticas de Saúde. Coordenação Nacional de DST e AIDS. Controle de infecções e a prática odontológica em tempos de AIDS: manual de condutas.
Brasília: Ministério da Saúde; 2000 [citado 2011 Jul 15]. Disponível em: < http://bvsms.saude.gov.br/bvs/publicacoes/manual_condutas_hepatite_hiv.pdf>.
10. Tortora GJ, Funke BR, Case CL. Microbiologia. Porto Alegre: Artmed; 2005. [ Links ]
11. Oliveira CAS, Costa SM, Zocratto KBF, Branco KMGR. Avaliação microbiana da recontaminação de artigos odontológicos estéreis segundo o manuseio das embalagens. Rev Facul Odontol. 2011;16(3):256-60. [ Links ]
12. Brasil. Ministério da Saúde. Resolução SS-27, de 28 de fevereiro de 2007. Aprova Norma Técnica que institui medidas de controle sobre o uso do Glutaraldeído nos Estabelecimentos Assistenciais de Saúde. Diário Oficial do Estado de São Paulo, São Paulo; 2007 Abr 18; Seção 1. p. 1-21 [citado 2011 Jul 15]. Disponível em: <http://www.hemocentro.fmrp.usp.br/projeto/legislacao/SS-27-REP_280207.pdf> [ Links ].
13. Ferreira EC, Ferreira IRC, Sanmartin JA, Verotti MP, Martins ST. Fluxo e processamento de artigos. Brasília: Agência Nacional de Vigilância Sanitária; 2006. p. 75-87. [ Links ]
14. Sociedade Brasileira de Enfermeiros de Centro Cirúrgico (SOBECC). Recuperação anestésica e centro de material e esterilização: práticas recomendadas da SOBECC. 2ª ed. São Paulo: SOBECC; 2003. [ Links ]
 Correspondence to:
Correspondence to:
AP NARDO
Rua Alexandre Guimarães, 1927, Areal, 78916-450, Porto Velho, RO, Brasil
e-mail: anapaulanardo@hotmail.com
Received on: 24/7/2011
Final version resubmitted on: 9/7/2012
Approved on: 28/11/2012













