Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.1 Porto Alegre Jan./Mar. 2013
ORIGINAL / ORIGINAL
Analysis of the polymorphism of osteoprotegerin by polymerase chain reaction in patients with type 2 diabetes and the association with periodontal condition
Análise de polimorfismos da região promotora T/C950 da osteoprotegerina através da reação em cadeia de polimerase em pacientes diabéticos tipo 2 e com periodontite
Mariana de Moraes Corrêa PEREZI; Keila Cristina Raposo LUCENAI; Paulo Roberto Eleutério de SOUZAII; Renata CIMÕESI; Jair Carneiro LEÃOI; Alessandra de Albuquerque Tavares CARVALHOI
I Universidade Federal de Pernambuco, Curso de Odontologia
II Universidade Federal Rural de Pernambuco, Curso de Ciências Biológicas. Recife, PE, Brasil
ABSTRACT
Objective
The aim of this paper was to analyze the presence of polymorphism in the promoter region T/C950 of the osteoprotegerin gene and its distribution in diabetic patients with periodontitis, when compared to the control group.
Methods
67 patients took part in the research. The test group (n = 32) was composed of diabetic patients with periodontitis and the control group (n = 35) included patients without diabetes and without periodontitis. For the diagnosis of periodontitis, the following clinical parameters were evaluated: probing depth, bleeding on probing and clinical attachment level. The DNA to investigate the polymorphisms of osteoprotegerin, obtained through the technique of polymerase chain reaction, was obtained from the blood serum of the participants.
Results
Polymorphisms of osteoprotegerin were found in promoter region -950T/C but there was no significance (p=1.000). Only the control group showed significant results for the probing depth according to the polymorphic region.
Conclusion
No influence was found between genetic polymorphisms of osteoprotegerin in patients with diabetes and periodontitis.
Indexing terms: Diabetes mellitus. Genetic polymorphism. Osteoprotegerin. Periodontitis.
RESUMO
Objetivo
Analisar a presença de polimorfismos na região promotora T/C950 do gene da osteoprotegerina, e a sua distribuição em pacientes diabéticos e com periodontite, quando comparados ao grupo controle saudável.
Métodos
A pesquisa contou com a participação de 67 indivíduos distribuídos em um grupo teste (n=32), constituído por pacientes diabéticos e com periodontite, e um grupo controle (n=35) que incluía pacientes não diabéticos e sem periodontite. Para o diagnóstico da periodontite, foram avaliados os parâmetros clínicos: profundidade de sondagem, sangramento à sondagem e nível de inserção clínica. O DNA para a investigação dos polimorfismos da osteoprotegerina, através da técnica da reação em cadeia de polimerase convencional, foi obtido a partir de amostras sanguíneas dos participantes.
Resultados
Polimorfismos no gene da osteoprotegerina foram encontrados na posição -950T/C da região promotora, porém sem significância estatística (p=1,000). Apenas o grupo controle apresentou resultados significativos para a profundidade de sondagem segundo a região polimórfica (p=0,017).
Conclusão
Não foi observada influência entre o polimorfismo da região T/C950 do gene da osteoprotegerina em pacientes com periodontite e a diabetes mellitus.
Termos de indexação: Diabetes mellitus. Polimorfismo genético. Osteoprotegerina. Periodontite.
INTRODUCTION
A number of studies that have been carried out believe that diabetes has a big influence on the onset and progression of periodontal disease which, in turn, also influences glycemic control and that there exists, therefore, a bidirectional relationship between the two diseases in which diabetes predisposes the establishment of periodontal disease and the latter has a negative influence on the metabolic control of diabetes1-2.
The prevalence and severity of periodontal disease in diabetics has been shown to be greater than that of the population as a whole3-4, where the effects of periodontal disease in these individuals are aggravated by metabolic and histopathological changes, characteristics of diabetes, as well as vascular changes, defective immunological responses and a delayed process of healing1,5-7.
One of the main characteristics of periodontitis is alveolar bone resorption. Both the evaluation of the loss of periodontal clinical attachment level and the presence of periodontal pockets are fundamental to the diagnosis of this pathological condition. However, new forms of diagnosis are being used to evaluate the onset and progression of periodontitis, such as the identification of
concentrations of inflammatory mediators, such as the interleukins, as well as the analysis of specific mediators of bone metabolism, following the example of the activating receptor of nuclear factor Kappa B (RANK), its ligand RANKL and osteoprotegerin (OPG)8-11.
Osteoprotegerin, also known as osteoclastogenesis inhibitory factor, works as a receptor activator of nuclear factor kappa-B ligand (RANKL), having the ability to reduce bone resorption by acting in competition with the RANK, a surface receptor expressed by osteoclasts12-13. The RANKL, by binding to the RANK, activates differentiation, increases activity and inhibits the apoptosis of the osteoclasts. Nevertheless, these biological effects are the opposite when the RANKL binds to osteoprotegerin, by inhibiting osteoclastogenic activity9,14-16.
As periodontal disease and diabetes are linked to bone resorption, the OPG/RANK balancing mechanism has also been investigated in both pathologies17-19. Differences in the proportion of these mediators may be connected to the severity of periodontal disease which could serve as a biomarker suggesting the occurrence of periodontitis20. Moreover, the presence of genetic polymorphisms in the protein osteoprotegerin is capable of establishing changes in the genetic code that could affect the phenotype, increasing susceptibility to certain diseases6,12.
One study that was carried out to check for the occurrence of polymorphisms in osteoprotegerin21, confirmed the presence of 12 different polymorphisms in this gene, including that of position -950T/C in the promoter region. The identification of this polymorphism could help with the diagnostic evaluation of the risk of periodontitis. The aim of the present study, therefore, was to analyze the presence of polymorphisms in the T950C promoter region of the osteoprotegerin gene and its clinical impact on patients with diabetes and periodontitis, by making comparisons, systemically and periodontally, with the healthy control group.
METHODS
Sample
The present study included a sample of 67 patients of both sexes, aged between 22 and 56, and who had at least 15 teeth. Being an experimental study, it was not necessary to perform sampling calculations. The patients were cared for in the Ermírio de Moraes Medical Center in the city of Recife which is located in the Brazilian state of Pernambuco and also at the Stomatology Clinic at the Federal University of Pernambuco. The participants were divided into two groups: the test group, comprising 32 individuals with type 2 diabetes and with periodontitis and the control group, comprising 35 individuals without diabetes or periodontitis. Patients were considered to be diabetic when presenting with glucose levels in excess of 125mg/dL and glycated hemoglobin above 6.5%.
Excluded from the study were individuals under the age of 18, individuals with less than 15 teeth, smokers, women who were pregnant or breastfeeding, individuals on antibiotics, those that had been subjected to periodontal treatment within the previous 6 months as well as individuals undergoing medical treatment for systemic conditions, except diabetes.
The participants filled out a personal questionnaire and, after being informed of the nature of the study, signed a free and informed consent agreement, the study being conducted in compliance with the ethical principles involving research on human beings, as per the Helsinki declaration, and with the approval of the Ethics in Research Committee at the Federal University of Pernambuco, filed under case no. 088/09.
Clinical parameters evaluated
The diagnosis of periodontitis was made in accordance with the patient's periodontal clinical andradiographic examination. The periodontal clinical examinations were carried out by just one calibrated examiner, using a Williams type of manual millimeter probe (Trinity®, São Paulo, Brazil). Six areas of each tooth were examined (mesial-, mid- and disto-vestibular; mesial-, midand disto-palatal or lingual), except for the third molars, by evaluating the following clinical parameters: probe depth (distance between the gum margin and the most apical part of the pocket or groove, measured in millimeters), bleeding on probing (the presence/absence of bleeding was recorded after 30 seconds of probing at depth had elapsed, with respective scores of 1 and 0) and the clinical attachment level (a measurement corresponding to the distance from the amelocemental junction to the most apical part of the periodontal pocket or groove, obtained through the sum of the measurements of probe depth and gingival recession). Patients were considered to have periodontitis when having a probe depth greater than or equal to 4 mm in more than one area examined, as per the criteria established by the American Academy of Periodontology (AAP).
Laboratory analysis using the PCR technique
DNA was extracted from the participants' blood samples using the DNA blood mini kit (Qiagen) in compliance with manufacturer's instructions. In order to carry out the technique of Polymerase Chain Reaction (PCR), a fragment with 348 base pairs (bp) corresponding to the promoter region of the OPG gene was amplified using the conventional PCR technique, in a total volume of 25μL containing 12.5 μL of mix (Top Taq Polymerase - Qiagen), 0.6 μL of primer (F- GTTCCTCAGCCCGGTGGCTTTT and R- TGTGGTCCCCGGAAACTTCAGG) and 4 μL of DNA. The amplifications were carried out through 35 denaturation cycles at 96°C for 45 seconds; annealing temperature of 64°C for 45 seconds; extension at 72°C for 45 seconds with initial denaturation at 96°C for 5 minutes and final extension at 72°C for 10 minutes, as per Park et al.5. The RFLP technique (detection of the length of the amplified fragments after digestion by the restriction enzyme) of the T/C950 region was performed using the HincII restriction enzyme (Promega), through which the products of the PCR were digested at 37°C for 2 hours and separated in 3% agarose gel.
Statistical analysis
The data were analyzed using the software application Statistical Package for the Social Sciences (SPSS) version 15.0, via descriptive and inferential statistical methods. The normality of data distribution was tested to check the adequacy of the statistical test (parametric or non-parametric). The prevalence of genotypes and alleles amongst the test group and the control group were analyzed using Pearson's chi-squared test (χ2) and Fisher's exact test. The association between the genotypes and the clinical parameters was verified using the Kruskall-Wallis test with comparison to the aforementioned test, and the F test (ANOVA) with Tukey comparisons, where the level of significance was assumed to be p<0.05.
RESULTS
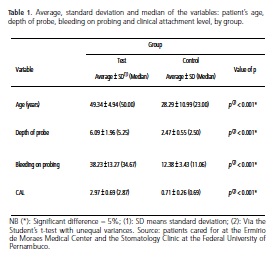
The average age of the patients in the test group was approximately 49, while in the control group it was approximately 28 (Table 1). The difference in age occurred because the clinical examination of the patients in the test group was performed in a diabetes treatment center where the age of the diabetic patients was higher. As for the variables probe depth, bleeding on probing and clinical attachment level, the averages were higher in the test group, presenting significant differences in comparison with the control group (Table 1).
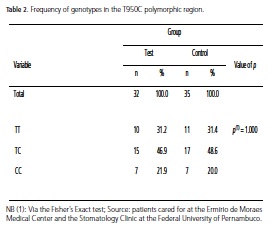
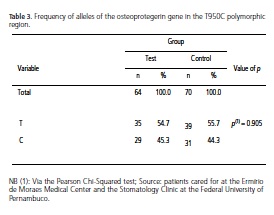
Based on the DNA sequencing and on the amplified fragment length detection technique applied after digestion by the restriction enzyme (PCR-RFLP), polymorphisms in the OPG gene were located in position 950T/C of the promoter region. These mutations were represented by the presence of two bands, characterizing a CC-homozygote carrier, however this mutation was not statistically significant (Table 2). The allele distribution, displayed in Table 3, also showed no significant results in terms of an association between periodontal disease and diabetes, although a greater prevalence of the T allele has been observed in both study groups.
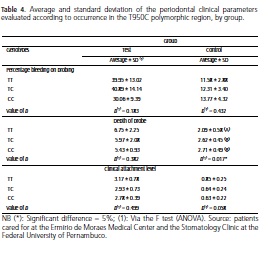
Table 4 compares the clinical parameters (probe depth, bleeding on probing and clinical attachment level) with the polymorphic regions in the two groups being studied so as to be able to evaluate a potential association between the severity of periodontal disease in type 2 diabetic patients and the presence of polymorphisms in the 950T/C region in the OPG gene. The depth of the probe in the control group presented significant differences, with lower averages in the TT genotype and higher and more approximate averages for the TC and CC (mutant) genotypes (p=0.017). As for bleeding on probing and clinical attachment level, no significant results were found.
DISCUSSION
Diabetes produces systemic changes that are capable of contributing to the onset and progression of periodontitis, a complex pathology heavily influenced by genetic factors22. The link between these two pathologies is quite well known in the literature by virtue of their high prevalence. The present study investigated the presence of genetic polymorphisms in the T950C promoter region of the OPG gene in the test and control groups and the possible influence of these on the clinical status of patients with type 2 diabetes and chronic periodontitis, but did not observe significant results with regard to such an influence.
Although the high association, high prevalence and incidence of periodontal disease in diabetic patients is well established in the literature, few studies have been conducted to identify genetic markers that might contribute to an increase in susceptibility to these diseases. The identification of genes that influence the establishment of periodontitis in diabetics could provide a risk assessment for the development of these two connected pathologies8. Differences in RANKL/OPG concentrations may be regarded as an important biomarker for periodontitis, with RANKL being a positive marker to the detriment of OPG which is a negative marker20.
One study23 evaluating the modulatory role of diabetes in patients with chronic periodontitis suggested that this modulation might occur by virtue of the inhibition of OPG production in diabetic patients, thereby favoring the process of local bone resorption. Other studies11,24, however, show that high levels of OPG could be found in diabetic patients due to dysfunction of the endothelial cells and a greater susceptibility of these patients to vascular diseases, where high levels of OPG could represent a defense mechanism against arterial calcification and other forms of vascular damage. However, according to other studies5,11, other aspects with the onset of periodontal disease should be remembered such as the influence of microbial factors and the existence of other bone metabolism markers.
As OPG is involved in the process of bone remodeling, polymorphisms in its genetic structure could exert a big impact on the structural and functional properties of this protein, altering the RANKL/OPG proportions and causing a predisposition to greater destruction of alveolar bone25. The aim of the present study was to analyze the possible influence of genetic polymorphisms of the T950C promoter region, of the aforementioned gene, between periodontitis and type 2 diabetes, in view of the scarcity of studies in the literature in respect of this association.
When analyzing the possible association of polymorphisms in the T950C region of the OPG with chronic periodontitis, several researchers8,12 found no significant results. Similarly, in our study, this association was not significant. However it should be stressed that, in the present study, patients were evaluated who had chronic periodontitis and type 2 diabetes in which the diabetes could exert a modulatory effect on the periodontitis.
Verifying other accounts, no association was found between aggressive26 and chronic periodontitis25 with the polymorphism in the OPG gene. The results of these studies confirm our findings in which the presence of OPG polymorphisms, chronic periodontitis and diabetes is associated. However, the latter study25 evaluated polymorphisms in the exons of the protein, in contrast to our study which evaluated the presence of these polymorphisms in the promoter region of the aforementioned protein.
Although the association of diabetes with periodontitis is bidirectional, to our knowledge, few reports exist in the literature on this association with the presence of genetic polymorphisms, in particular with OPG. Many studies, however, associate these polymorphisms with other diseases, such as osteoporosis14-16,21-22,26-28, rheumatoid arthritis27, renal problems12 and cardiovascular diseases9,13,15,27. In a study that set out to investigate the association between OPG polymorphisms, chronic periodontitis and renal problems12, no association was found in the T950C region, in the same way that our study did not find a statistical significance between the presence of genetic polymorphisms in this promoter region with type 2 diabetes and periodontitis. In our reports, however, the depth of probe index was seen to be statistically significant in the control group (p=0.017) which, as expected, had an average depth 3 times smaller than that of the test group. In addition, the highest frequency of genotypes in this group was that of the mutant genotype CC (2.71). Accordingly, it may be inferred that the presence of polymorphisms in this group, for this periodontal index, could represent a protecting effect against periodontitis. However the small sample size within the group studied must also be taken into consideration.
As OPG is a candidate gene for the genetic control of bone mass, some studies have analyzed the polymorphism in the OPG gene in patients with osteoporosis where several authors28 observed a positive association between the polymorphisms in the T950C promoter region of the OPG gene with a reduction in bone mineral density. Other studies14,22, however, found no significant results between the polymorphism in this region and osteoporosis, as is the case with our study which found no influence between this polymorphism and type 2 diabetes. Other authors16,21 observed an association of the CC genotype in the T950C region with a greater bone mineral density in some anatomical locations. However, even given this observation, the values were not significant.
Despite there being no consensus in the literature, several studies suggest an association of the T950 allele with an increase in osteoclastic activity, an essential characteristic of periodontal diseases. A higher frequency of the T allele in individuals with periodontitis was observed in our study, both in the test group and the control group. However its value was not statistically significant. This fact differs from those observed by other investigators8 who reported a higher prevalence and significance of the T950 allele in patients with periodontitis. However these investigators studied Koreans who were genetically homozygote, in contrast to our study group which comprised a genetically heterozygote population, like the population observed in another study12 which also found no significant influence between the aforementioned allele and chronic periodontitis.
An association of the polymorphism in the promoter region of the OPG in position -950T/C with morphology and vascular function was also reported22, where the genotype CC could be associated with vascular diseases, another characteristic related to periodontal diseases as well as type 2 diabetes mellitus. In the present study, the frequency of the mutant genotype CC was similar in both groups studied, without presenting statistically significant values. On the other hand, some studies9,22 found the genotype CC was related to an increase in the thickness of the intima-media layer of the common carotid artery, an early indication of atherosclerosis. Agreeing with these reports, other accounts29 have shown that the aforementioned genotype of the T950C region was higher in patients with atherosclerosis. As a complement to these observations, high frequencies of the mutant C allele in this polymorphic region were observed in individuals with aortic calcifications30, contrary to our findings where the frequency of this allele was lower, when compared to the wild T allele in both the groups studied. This information is quite interesting and relevant and should be the target of future investigations as chronic periodontal disease could be considered as an important risk factor for these groups of diseases as well as being associated with diabetes.
CONCLUSION
No clinical influence was found in relation to the presence of polymorphisms in the T950C region in the OPG gene with type 2 diabetes or with periodontitis. However further studies should be carried out with this aim in order to evaluate the involvement of bone metabolism mediators in the determination of susceptibility and the progression of periodontitis in diabetic patients.
Collaborators
MMC PEREZ and KCR LUCENA participated in the research study conceptualization and the composition of the article. PRE SOUZA and R CIMÕES contributed to the data analysis and composition of the article. JC LEÃO and AAT CARVALHO directed the research and participated in the composition of the article.
REFERENCES
1. Quirino MRS, Jardim JCM, Rezende PHN, Bulhões RC, Pallos D. Doença periodontal e diabetes mellitus: uma via de mão dupla. Rev Ciênc Med. 2009;18(5/6):235-41. [ Links ]
2. Alves C, Andion J, Brandão M, Menezes R. Mecanismos patogênicos da doença periodontal associada ao diabetes melito. Arq Bras Endocrinol Metab. 2007;51(7):1050-57. doi: 10.1590/S0004-27302007000700005. [ Links ]
3. American Academy of Periodontology. Position paper: diabetes and periodontal disease. J Periodontol. 2000;71(4):664-78. doi: 10.1902/jop.2000.71.4.664. [ Links ]
4. Mealey BL. Periodontal disease and diabetes: a two-way street. J Am Dent Assoc. 2006;137:S26-31. [ Links ]
5. Madeiro AT, Bandeira FG, Figueiredo CRLV. A estreita relação entre diabetes e doença periodontal inflamatória. Odontol Clín-Cient. 2005;4(1):7-12. [ Links ]
6. Hoçoya LS, Jardini MAN. Polimorfismo genético associadoà doença periodontal na população brasileira: revisão de literatura. Rev Odontol UNESP. 2010;39(5):305-10. [ Links ]
7. Cole CM, Sundararaj KP, Leite RS, Nareika A, Slate EH, Sanders JJ, et al. A trend of increase in periodontal interleukin-6 expression across patients with neither diabetes nor periodontal disease, patients with periodontal alone, and patients with both diseases. J Periodontol Res. 2008;43:717-22. doi: 10.1111/j.1600-0765.2010.01286.x. [ Links ]
8. Park OJ, Shin SY, Choi Y, Kim MH, Chung CP, Ku Y, et al. The association of osteoprotegerin gene polymorphisms with periodontitis. Oral Dis. 2008;14(5):440-4. doi: 10.1111/j.1601- 0825.2007.01398.x. [ Links ]
9. Brändström H, Stiger F, Lind L, Kahan T, Melhus H, Kindmark A. A single nucleotide polymorphism in the promoter region of the human gene for osteoprotegerin is related to vascularmorphology and function. Biochem Biophys Res Commun. 2002;293(1):13-7. doi: 10.1016/S0006-291X(02)00137-7. [ Links ]
10. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):1-7. doi: 10.1186/ar2165. [ Links ]
11. Lappin DF, Eapen B, Robertson D, Young J, Hodge PJ. Markers of bone destruction and formation and periodontitis in type 1 diabetes mellitus. J Clin Periodontol. 2009;36(8):634-41. doi: 10.1111/j.1600-051X.2009.01440.x. [ Links ]
12. Baioni CS, Souza CM, Ribeiro APB, Luczyszyn SM, Dias da Silva MA, Ignácio AS, et al. Analysis of the association of polymorphism in the osteoprotegerin gene with susceptibility to chronic kidney disease and periodontitis. J Periodont Res. 2008; 43(5):578-84. doi: 10.1111/j.1600-0765.2008.01098.x. [ Links ]
13. Soufi M, Schoppet M, Sattler AM, Herzum M, Maisch B, Hofbauer LC, et al. Osteoprotegerin gene polymorphisms in men with coronary artery disease. J Clin Endocrinol Metab. 2004;89(8):3764-8. doi: 10.1210/jc.2003-032054. [ Links ]
14. Arko B, Prezelj J, Komel R, Kocijancic A, Hudler P, Marc J. Sequence variations in the osteoprotegerin gene promoter in patients with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2002;87(9):4080-4. doi: 10.1210/jc.2002-020124. [ Links ]
15. Hofbauer LC, Schoppet M. Osteoprotegerin gene polymorphism and the risk of osteoporosis and vascular disease. J Clin Endocrinol Metab. 2002;87(9):4078-9. doi: 10.1210/jc.2002- 021063.16. Steinemann SG .The insult of foreign body. Eur Cells Mater. 2001; 1(1):1. [ Links ]
16. Vidal C, Brincat M, Xuereb Anastasi A. TNFRSF11B gene variants and bone mineral density in postmenopausal women in Malta. Maturitas. 2006;53(4):386-95. doi: 10.1016/j. maturitas.2005.11.003. [ Links ]
17. Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Töz H, Atilla G, et al. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007;34(5):370-6. doi: 10.1111/j.1600-051X.2007.01061.x. [ Links ]
18. Hie M, Shimono M, Fujii K, Tsukamoto I. Increased cathepsin K and tartrate-resistant acid phosphatase expression in bone of streptozotocin-induced diabetic rats. Bone. 2007;41(6):1045-50. doi: 10.1016/j.bone.2007.08.030. [ Links ]
19. Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560-8. doi: 10.1359/jbmr.070115. [ Links ]
20. Belibasakis GN, Bostanci N. The RANKL/OPG system clinical periodontology. J Clin Periodontol. 2012;39(3):239-48. doi: 10.1111/j.1600-051X.2011.01810.x. [ Links ]
21. Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF. Polymorphisms in the osteoprotegerin gene are associated with osteoporotic fractures. J Bone Miner Res. 2002;17(7):1245-55. doi: 10.1359/jbmr.2002.17.7.1245. [ Links ]
22. Brändström H, Gerdhem P, Stiger F, Obrant KJ, Melhus H, Ljunggren O, et al. Single nucleotide polymorphisms in the human gene for osteoprotegerin are not related to bone mineral density or fracture in elderly women. Calcif Tissue Int. 2004;74(1):18-24. [ Links ]
23. Duarte PM, Neto JB, Casati MZ, Sallum EA, Nociti FH Jr. Diabetes modulates gene expression in the gingival tissues of patients with chronic periodontitis. Oral Dis. 2007;13(6):594-9. doi:
10.1111/j.1601-0825.2006.01348.x.
24. Costa PP, Trevisan GL, Macedo GO, Palioto DB. Salivary interleukin-6, matrix metalloproteinase-8 and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol. 2010;81(3):384-91. doi: 10.1902/jop.2009.090510. [ Links ]
25. Wagner J, Kaminski WE, Aslanidis C, Moder D, Hiller KA, Christgau M, et al. Prevalence of OPG and IL-1 gene polymorphismin chronic periodontitis. J Clin Periodontol. 2007;34(10):823-7. doi: 10.1111/j.1600-051X.2007.01132.x. [ Links ]
26. Soedarsono N, Rabello D, Kamei H, Fuma D, Ishihara Y, Suzuki M, et al. Evaluation of RANK/RANKL/OPG gene polymorphisms in aggressive periodontitis. J Periodontal Res. 2006;41(5):397-404. doi: 10.1111/j.1600-0765.2006.00874.x. [ Links ]
27. Bramblia-Tapa AJ, Durán-González J, Sandoval-Ramirez L, Mena JP, Salazar-Páramo M, Gámez-Nava JI, et al. MTHRF C677T, MTHRF A1298C and OPG A163G polymorphisms in Mexican
patients with rheumatoid arthritis and osteoporosis. Dis Markers. 2012;32(2):109-14. doi: 10.3233/DMA-2011-0868.
28. Vidal C, Formosa R, Xuerea-Anastasis A. Functional polymorphisms within the TNFRS11B (osteoprotegerin) gene increase the risk for low bone mineral density. J Mol Endocrinol. 2011;47(3):327-33. doi: 10.1530/JME-11-0067. [ Links ]
29. Strafac G, Biscetti F, Pitocco D, Bertoletti G, Misuraca M, Vincenzoni C, et al. Assessment of the genetic effects of polymorphisms in the osteoprotegerin gene, TNFRS11B on serum osteoprotegerin levels and carotid plaque vulnerability. Stroke. 2011;42(11):3022-8. [ Links ]
30. Rhee EJ, Oh KW, Jung CH, Lee WY, Oh ES, Yun EJ, et al. The relationship between four single nucleotide polymorphisms in the promoter region of the osteoprotegerin gene and aortic calcification or coronary artery disease in Koreans. Clin Endocrinol (Oxf). 2006;64(6):689-97. doi: 10.1111/j.1365-2265.2006.02530.x. [ Links ]
 Correspondence to:
Correspondence to:
RM VIEIRA
Av. Prof. Moraes Rego, 1235, Cidade Universitária, Recife, PE, Brasil
e-mail: ronanmvieira@gmail.com
Received on: 20/3/2012
Final version resubmitted on: 21/8/2012
Approved on: 9/10/2012













