Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.2 Porto Alegre Abr./Jun. 2013
ORIGINAL / ORIGINAL
Assessment of new bone formation around titanium surface treated implants in diabetic rats
Neoformação óssea ao redor de implantes de titânio com superfície tratada inseridos em ratos diabéticos
Gabriela GENNAROI; Gerson Francisco de ASSISI; Tânia Mary CESTARII; Rubens RODRIGUES FILHOII
I Universidade de São Paulo, Faculdade de Odontologia, Departamento de Biologia Oral. Alameda Dr. Octávio Pinheiro Brisolla, 9-75, Vila Universitária, 17012-901, Bauru, SP, Brasil.
II Universidade Federal de Santa Catarina, Centro de Ciências da Saúde, Faculdade de Odontologia, Departamento de Estomatologia. Florianópolis, SC, Brasil.
ABSTRACT
Objective
This study aimed to compare the bone formation around titanium implants with machined and acid-etched surfaces, inserted in induced diabetic rats and in non-diabetic rats, in an attempt to investigate whether there are differences in bone formation between this metabolic condition and the use of different implant surfaces.
Methods
Custom fabricated commercially pure solid cylinder titanium implants, machined and acid-etched surface were inserted in the femora of streptozotocin-induced diabetic rats (n=10) and non-diabetic rats (n=10). Morphometrical bone-implant contact percentage and bone area within the limits of the implant threads (BD) were performed at 21 days of healing.
Results
Peri-implant tissue in machined implant showed intense new bone formation within all threads of the implants of the non-diabetic group (BIC = 82.8 ± 9.23 e BD = 38.7 ± 4.27) while diabetic group (BIC = 35.3 ± 9.4 and BD = 20.0 ± 3.8) exhibited small and immature bone formation within threads of the implants with thickness fibrous connective tissue interposition between bone-implant interface. In the acid-etched surface implants in both, diabetic and non-diabetic groups, the peri-implant tissue showed intense new bone formation within all threads of the implants with BIC = 74.4 ± 14.7 e BD = 35.4 ± 3.48 in non-diabetic group and BIC = 63.1 ± 12.9 e BD = 29.6 ± 4.9 in diabetic group.
Conclusion
In machined surface implants the diabetes interfere negatively in osseointegration while acid-etched surface promoted major BIC and BD index, indicating its selective use in diabetic patients.
Indexing terms: Dental implantation. Diabetes mellitus. Osseointegration.
RESUMO
Objetivo
Comparar a formação óssea ao redor de implantes de superfície lisa e tratada, instalados em ratos diabético-induzidos e não-diabéticos, investigando se há diferenças na formação óssea entre os dois quadros metabólicos, melhora no padrão de osteogênese entre as diferentes superfícies e, sua relação com o diabetes.
Métodos
Foram instalados implantes de titânio de superfícies lisa e tratada, no fêmur de ratos diabético-induzidos com estreptozotocina (n=10) e não diabéticos (n=10).A Análise morfométrica da porcentagem de contato osso-implante (COI) foi realizada 21 dias após a cirurgia.
Resultados
A neoformação óssea foi intensa ao redor dos implantes de superfície lisa nos ratos não-diabéticos (COI = 82.8 ± 9.23), enquanto que o grupo diabético exibiu pequena e imatura formação óssea (COI=35.3 ± 9.4), com interposição de tecido conjuntivo na interface osso-implante. Ao redor dos implantes com superfície tratada, ocorreu intensa neoformação óssea, tanto nos animais diabéticos (COI = 63.1 ± 12.9) como nos não-diabéticos, (COI = 74.4 ± 14.7).
Conclusão
Nos implantes de superfície lisa, o diabetes interfere negativamente na osseointegração, enquanto que as superfícies tratadas com ácido promoveram maior contato osso-implante, indicando seu uso seletivo em pacientes diabéticos.
Termos de indexação: Implantação dentária. Diabetes mellitus. Osseointegração.
INTRODUCTION
Diabetes, classified into type I and II, is associated with many complications that increase morbidity and mortality in affected individuals. Such complications result from an abnormal regulation of glucose metabolism, due to the absence or reduction in insulin production or resistance to it1.
Many changes in bone metabolism associated with diabetes can consequently affect the process of osseointegration of dental implants. Insulin acts directly or indirectly in the synthesis of bone matrix and in bone metabolism. It directly stimulates the synthesis of bone matrix, and indirectly induces production of Insulin-Like Growth Factor (IGF-I) by the organism. IGF-I regulates the synthesis of bone matrix by two mechanisms: it increases the number of osteoblasts and controls the function of osteoblast differentiation2. In vitro experiments indicate that topical application of insulin stimulates the process of intramembranous ossification in fractures3, increases the osteoid matrix area and the number and survival of osteoblasts, reducing their level of apoptosis. Also, another in vitro study verified that hyperglycemia increased proliferation and inhibited mineralization of cultured osteoblasts, while IGF-I has reverted that condition4.
Several histomorphometric studies investigating osseointegration around implants inserted in the femur or tibia have shown that in streptozotocin-induced diabetic rat model, the bone-to-implant contact is impaired5-7. Titanium implants have also been placed in the maxillary molar socketsof a type 1 diabetic rat model, where the mineral apposition rate was reduced, although no morphological differences in bone structure were evident once the bone healing process had finished8.
The biological response after implantation determines the quality and speed of the bone-to-implant healing process. The interaction of the implant with its biological environment, the formation of the implant material-tissue interface, and the long-term outcome of the implant integration are strongly dependant on the physicochemical properties of the implant surface9. As an attempt to favor osteogenesis, especially in patients who have unfavorable systemic conditions, the surface topography of implants has been changed. There is, however, a continuing drive in terms of implant design to develop improved titanium surfaces which osseointegrate faster and with greater efficiency. Their clinical aim is to promote even greater long-term implant success, in addition to reducing the risk of complications, particularly in medically compromised patients at risk of impaired bone healing with conditions such as diabetes and osteoporosis. Many studies6,10-12 have shown that a rough surface, when compared to a relatively smooth one, grants better biomolecular adsorption, increases extracellular matrix production and promotes the differentiation of the mesenchymal cells toward an osteoblastic phenotype . So various surface treatment techniques such as chemical treatment, mechanical treatment and deposition methods were employed in order to modify titanium surface for improving its bioactivity9,12. Chemical surface modification of titanium implant is particular useful. Etching with strong acids such as HCl, H2SO4, HNO3 and HF is another method for roughening titanium dental implants. It has been known that acid etched surfaces improve the osteoconductive process due to the attachment of osteogenic cells. As a result, bone cell is formed on the surface of the implant directly13. It is obvious that bone growths into porous implant surfaces improve osseointegration and mechanical stability by the interlock between surrounding bone tissue and implant9,13, so in unfavorable clinical situations, early and high bone-to-implant contact would be beneficial to allow high levels of loading14-15.
This study aimed to compare the bone formation around implants with machined and acid-etched surfaces, inserted in femur of induced diabetic and non-diabetic rats, investigating whether there are differences in bone formation between both metabolic conditions, improvement in osteogenesis pattern between different surfaces and their relation with diabetes.
METHODS
The procedures performed in this experiment were approved by the Animal Ethics Committee (CEUA) at the Federal University of Santa Catarina, protocol n. PP00094.
Animals
The study was conducted on twenty male Wistar rats, aged approximately 3 months with a mean weight of 350 g, maintained under controlled environment conditions (ambient temperature 22 ± 2oC and light/dark 12h/12h periods) and free access to water and food. The animals were divided into two groups: diabetic (n = 10) and non-diabetic (n = 10).
Induction of diabetes
Diabetes was induced by a single intraperitoneal injection of streptozotocin (70 mg/kg body wt, Sigma- Aldrich, St Louis, MO, USA) in 0.9% NaCl, in the animals of the diabetic group. The diabetic condition was confirmed seven days later by measurement of tail blood glucose (BG) level, using a glucose oxidase-impregnated test strip and a reflectance meter (Accu-Chek Active, Roche, Germany) and monitored weekly after surgery .Rats were considered diabetics when, their plasma glucose levels were greater than 11.11mmol/L.
Surgical protocol for implant insertion
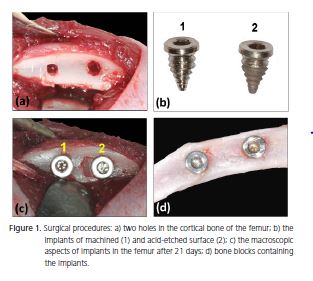
All surgical procedures were performed using aseptic techniques, under deep anesthesia by intraperitoneal injection of 7% chloral hydrate solution (0.6 ml/100 g of body weight). A longitudinal incision was made on the medial surface of the femur and the bone diaphysis was exposed by blunt dissection. Two holes (Figure 1a) were made through the cortical bone, trabeculae and bone marrow bilaterally at a 5-mm distance, using a hand drill with a screw rod of 2.0-mm diameter and 4.0-mm height. This procedure was performed under extensive washing and cooling with sterile 0.9% NaCl to remove bone debris. Implants (SysthexBrasil, Curitiba, Brazil) with machined and treated (acid-etched) surfaces were inserted in the holes (Figure 1b-c).
On the immediate postoperative period, 0.06 ml/ Kg of benzathinebenzylpenicillin (Benzetacil 1.200.000 IU, EurofarmaLaboratórios Ltda.) was administered at a single intramuscular dose and 10 mg/Kg acetaminophen was used as analgesic (Paracetamol, EurofarmaLaboratórios Ltda.), diluted in consumption water for three consecutive days.
Histological procedures
The rats were sacrificed with a lethal dose of anesthetics at 21 days after surgery. Bone blocks containing the implants (Figure 1d) were removed and fixed in 10% formaldehyde for one week. After fixation, they were demineralized in Morse's solution (50% formic acid and 20% sodium citrate, in a 1:1 ratio) for 15 days, dehydrated in ethanol, cleared with xylene and embedded in HistosecTM (Merck KGaA, Damstadt, Germany). Semi-serial 5-μm thick sections were obtained and stained with hematoxylin and eosin.
Histomorphometric analysis
Histomorphometry of bone-implant contact percentage (BIC), defined as the length of bone surface border in direct contact with the implant perimeter (100%), as well as the bone area within the limits of the implant threads (BD), was performed with a light microscope (Zeiss Axioskop 2, Germany) connected to a high-resolution video camera (Sony CCD-IRIS-RGB, Sony Corporation, Tokyo, Japan) and interfaced with a monitor and personal computer. This optical system was associated with a digitizing and a morphometry software package with image-capturing capabilities (Kontron Elektronik GmbH, Image Analysis Division, Echinf, Munich, Germany). In four semi-serial longitudinal sections, 40 fields per animal (10 histological fields/per section) selected by systematic sampling were obtained of each surface connected to the screw thread (distal and mesial), using 40X immersion objective. In these images the BIC and amount of bone area within the threads (from the lowest point of the microimplant head to the last apical thread) were calculated and expressed as BIC% and BD%, respectively.
Statistical analysis
Two-way ANOVA complemented by the Bonferroni's test for multiple comparisons was used for statistical analysis of differences between surfaces (machined and acid-etched) and conditions (diabetic and non-diabetic), at a significance level of p < 0.05.
RESULTS
Laboratory analysis
The diabetic status (blood glucose above 11,11mmol/L) was predictably induced and maintained over the experimental periods. In the diabetic group, the glycemic index did not show statistical differences (p>0.05) during the 21 days post-surgery with value mean of 24.11 ± 10.22 mmol/L. Also The blood glucose of non-diabetic group did not show statistical differences (p>0.05) during all experimental periods and glycemic index mean was of 5.72 ± 0.66 mmol/L.
As expected, non-diabetic rats had an increase in body mass of 23.75 ± 8.6 g at 21 days. Conversely, diabetic rats had a body weight loss of 67.75 ± 14.8 g at 21 days after surgery.
Histological analysis
Concerning the surgical aspect, all rats tolerated the implant placement well. On gross dissection, all implants were present in bone without apparent infection or inflammation and were immobile.
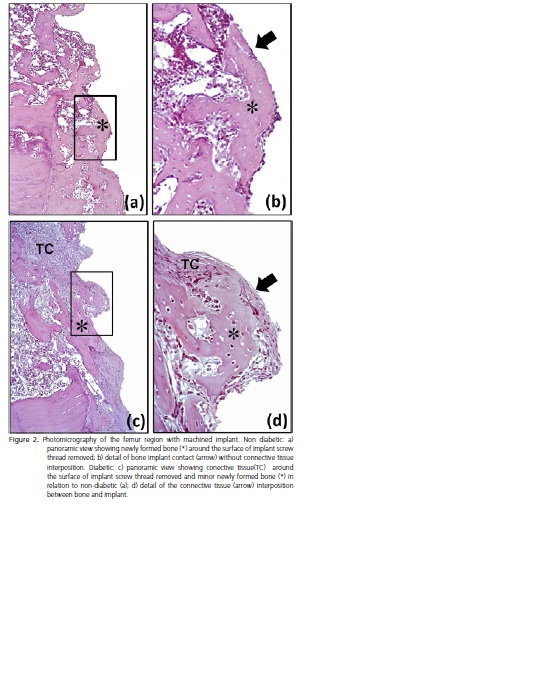
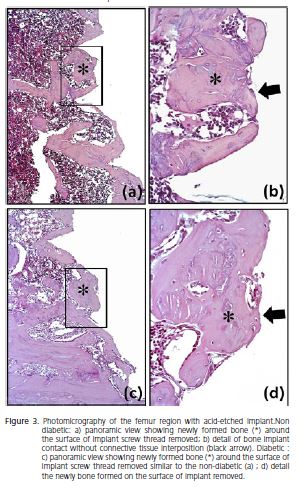
Histological analysis of the peri-implant tissue in machined implant showed intense new bone formation within all threads of the implants of the non-diabetic group (Figure 2a-b), while diabetic group exhibited small and immature bone formation within threads of the implants with thickness fibrous connective tissue interposition between bone-implant interface (Figure 2c-d).
The peri-implant tissue in all implants with acideatched surface in both, diabetic and non-diabetic groups showed intense new bone formation within all threads of the implants (Figures 3a-b and 3c-d), similar to observed in the machined implant of the non-diabetic group (to compare Figure 3a-b and 3c-d with 2a-b).
Morphometric analysis
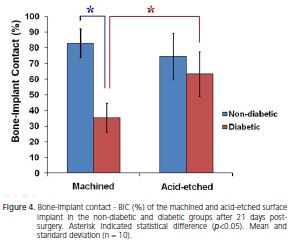
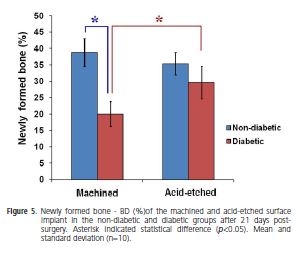
The percentage data observed in the implant interface compared according to the different conditions (diabetic versus non-diabetic) and implant surfaces (machined versus acid-etched) were presented in Figures 4 and 5.
Comparison between conditions showed that in the machined implants the BIC and BD were 2.34 and 1.94 folds higher, respectively, for non-diabetic than diabetic rats. The acid-etched surface did not show statistical differences between conditions (p<0.05).
Comparison between implant surfaces showed that in diabetic rats the BIC and BD for acid-etched surface were 1.80 and 1.48 folds higher, respectively, in relation to machined surface. However in non-diabetic group, BIC and BD in both surfaces did not show statistical differences (p>0.05). Thus, it was possible to observe that the acidetched surface improved the bone formation in diabetic rats.
DISCUSSION
Diabetic symptoms presented by streptozotocininduced diabetic rats are well established16. In this study, diabetic rats had weight loss, while control rats kept gaining body mass during the experiment. These results are in accordance with other studies5,7,17.
Induction of diabetes offers an adverse systemic environment, characterized by high levels of blood glucose. This results in impaired healing associated with microvascular and cellular disorders. Also, there are typical changes in bone healing, including reduction in osteogenesis, less cell renewal, formed bone with low mineral density and delay in healing of fractures18.
This study reports the effects of experimental diabetes on bone healing around implants with machined and etched surfaces. Thus, it was possible to observe that there were differences in bone formation between diabetic and non-diabetic rats at healing period of 21 days solely around machined surface implants.In this type of implant, the amount of newly formed bone (BD) and bone-implant contact (BIC) were significantly higher in the non-diabetic group than diabetic group.
The early failure of implants results from an inability to establish an intimate bone-to-implant contact19, being the bone healing after implant insertion delayed or even impaired. In fact, the mechanisms that normally lead to wound healing (i.e. bone apposition) do not take place; rather, a fibrous scar tissue is formed between the implant surface and surrounding bone. Indeed, our results suggested that failure of osseointegration of the machined implants in diabetic rats due to presence of fibrous connective tissue interposition at the interface between bone and implant. Consequently, connective tissue formation compromised the anchoring function of the endosseous implant, as previously described. Accordingly, both systemic and local factors can interfere with these primarily cellular events, playing prominent roles in early failures19,20-21.
It is worth noting that during implant retrieval for histological analysis, the risk of detaching bone fragments is high, and therefore, the bone-implant contact extension can be underestimated22-23. To address this problem, identical screw retrieval procedures were executed in both experimental and control groups; however, the results of this assay should be interpreted cautiously23.
Osseointegration of titanium dental implants is a complex phenomenon depending on several factors and characterized by a long sequence of events, such as the homing of multipotent mesenchymal cells, a cell proliferation phase coupled with the expression of some proteins, the induction of genes related to maturation and organization of bone extra cellular matrix, and finally a matrix mineralization phase24. Different treatments of titanium surface can modify the implant microstructural properties that in turn are able to affect bone formation processes24-25. Superficial morphology properties may play a critical role in biomolecular adsorption and cell adhesion to the implant surface as well as in osteoblast cells maturation11,24,26. However, optimal surface topography and roughness are still controversial.
Several experimental studies have reported higher bone-to-implant contact, less bone resorption and higher resistance to torque removal on acid-etched surfacescompared to manufactured surfaces15,27-28. Recently, acid-etching methods have been improved in order to increase cell adhesion and bone formation directly on the implant surface29. Accordingly, the acidetched topography seems to affect the behavior of human osteoblast-like cells determining a significant change in cell adhesion and modifying cellular shape and aggregation. It is therefore likely that specific surface properties of acidetched titanium implants may modulate the biological osteoblast behavior during bone tissue healing24.
Conversely, diabetes seems to increase the incidence of early failures and to delay the osseointegration process. Thus, in the presence of such disease, the choice of osseointegrated implants should eventually be made considering the use of titanium implants with treated surfaces for enhancing peri-implant bone healing in unfavorable clinical situations15,18. In accordance, Bugea et al.11 affirmed that osseointegration can be obtained when implants with a dual-acid-etched surface are placed in properly selected diabetic patients. In this way, our study showed that acid-etched surface improved the bone implant contact (BIC) and newly formed bone (BD) in diabetic rats, providing superior osseointegration in relation to the machined surface implants.
CONCLUSION
Regarding to difference in the amount of newly formed bone around machined implants in diabetic versus non-diabetic rats, it was concluded that bone formation impairment was probably caused by the diabetes and its implications in bone metabolism, but this does not seem to represent a definite contraindication to oral implantology. In addition, acid-etched surface implants were better inducers of new bone formation, even when associated with diabetes.
Collaborators
G GENNARO responsible for the overall work. Developed throughout the experimental part; induced animals with diabetes, followed daily course of the disease and installed implants in the femur of rats. Was responsible for the sacrifice of animals, histological processing of parts, microscopic analysis of the same, completion of the work and writing of the article. GF ASSIS co-mentored research development, contributing mainly to the histological stage and participated in writing the article. TM CESTARI collaborated with processing parts, guided the analysis of the blades, made and helped complete statistical analysis and interpretation of data and participated in writing the article. R RODRIGUES FILHO supervised the research and participated in the writing of the article.
REFERENCES
1. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11-63. doi: 10.2337/dc12-s011. [ Links ]
2. Fiorellini JP, Nevins ML, Norkin A, Weber HP, Karimbux NY. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin Oral implants Res. 1999;10(5):362-8. doi: 10.1111/ j.1600-0501.1999.tb00011.x.
3. Yano H, Ohya K, Amagasa T. Insulin enhancement of in vitro wound healing in fetal rat parietal bones. J Oral Maxillofac Surg. 1996;54(2):182-6. doi: 10.1016/S0278-2391(96)90444-9.
4. Fang Y, Wang ZY, Mao Y, Xin HT, Ren GL, Bai XF. Effects of insulin-like growth factor I on the development of osteoblasts in hyperglycemia. Diabetes Res Clin Pract. 2006;73(1):95-7.
5. Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003;12(3):242-51.
6. Kwon PT, Rahman SS, Kim DM, Kopman JA, Karimbux NY, Fiorellini JP. Maintenance of osseointegration utilizing insulin therapy in a diabetic rat model. J Periodontol. 2005;76(4):621- 6. doi: 10.1902/jop.2005.76.4.621.
7. McCracken MS, Aponte-Wesson R, Chavali R, Lemons JE. Bone associated with implants in diabetic and insulin-treated rats. Clin Oral Implants Res. 2006;17(5):495-500. doi: 10.1111/j.1600- 0501.2006.01266.x.
8. Shyng YC, Devlin H, Ou KL. Bone formation around immediately placed oral implants in diabetic rats. Int J Prosthodont. 2006;19(5):513-4.
9. Zareidoost A, Yousefpour M, Ghaseme B, Amanzadeh A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J Mater Sci Mater Med. 2012;23(6):1479-88. doi: 10.1007/s10856-012-4611-9.
10. Annunziata M, Oliva A, Basile MA, Giordano M, Mazzola N, Rizzo A, et al. The effects of titanium nitride-coating on the topographic and biological features of TPS implant surfaces. J Dent. 2011;39(11):720-8. doi: doi: 10.1016/j. jdent.2011.08.003.
11. Bugea C, Luongo R, Di Iorio D, Cocchetto R, Celletti R. Bone contact around osseointegrated implants: histologic analysis of a dual-acid-etched surface implant in a diabetic patient. Int J Periodontics Restorative Dent. 2008;28(2):145-51.
12. Le Guehennec L, Goyenvalle E, Lopez-Heredia MA, Weiss P, Amouriq Y, Layrolle P. Histomorphometric analysis of the osseointegration of four different implant surfaces in the femoral epiphyses of rabbits. Clin Oral Implants Res. 2008;19(11):1103- 10. doi: 10.1111/j.1600-0501.2008.01547.x.
13. Ramis JM, Taxt-Lamolle SF, Lyngstadaas SP, Reseland JE, Ellingsen JE, Monjo M. Identification of Early Response Genes to Roughness and Fluoride Modification of Titanium Implants in Human Osteoblasts. Implant Dent. 2012;21(2):141-9. doi: 10.1097/ID.0b013e31824a06b4.
14. Colombo JS, Balani D, Sloan AJ, Crean SJ, Okazaki J, Waddington RJ. Delayed osteoblast differentiation and altered inflammatory response around implants placed in incisor sockets of type 2 diabetic rats. Clin Oral Implants Res. 2011;22(6):578-86. doi: 10.1111/j.1600-0501.2010.01992.x.
15. Schlegel KA, Prechtl C, Most T, Seidl C, Lutz R, von Wilmowsky C. Osseointegration of SLActive implants in diabetic pigs. Clin Oral Implants Res. In press 2011.
16. Wong KK, Tzeng ES. Appearance of different diabetic symptoms after streptozocin administration: a comparison study. Biochem Molecular Biol Int. 1993;30(6):1035-41.
17. Claudino M, Ceolin DS, Alberti S, Cestari TM, Spadella CT, Rubira-Bullen IR, et al. Alloxan-induced diabetes triggers the development of periodontal disease in rats. PLoS One. 2007;2(12):e1320.
18. Alsaadi G, Quirynen M, Komarek A, van Steenberghe D. Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res. 2008;19(7):670-6. doi: 10.1111/j.1600-0501.2008.01534.x.
19. Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci. 1998;106(3):721-64. doi: 10.1046/j.0909-8836..t01-6-.x.
20. van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M. The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clin Oral Implants Res. 2002;13(6):617-22. doi: 10.1034/j.1600- 0501.2002.130607.x.
21. van Steenberghe D, Quirynen M, Molly L, Jacobs R. Impact of systemic diseases and medication on osseointegration. Periodontol 2000. 2003;33(1):163-71. doi: 10.1046/j.0906- 6713.2003.03313.x.
22. Nikolidakis D, Meijer GJ, Oortgiesen DA, Walboomers XF, Jansen JA. The effect of a low dose of transforming growth factor beta1 (TGF-beta1) on the early bone-healing around oral implants inserted in trabecular bone. Biomaterials. 2009;30(1):94-9. doi: doi: 10.1016/j.biomaterials.2008.09.022.
23. Munhoz EA, Bodanezi A, Cestari TM, Taga R, Ferreira Junior O, de Carvalho PS. Biomechanical and microscopic response of bone to titanium implants in the presence of inorganic grafts. J Oral Implantol. 2011;37(1):19-25. doi: 10.1563/AAIDJOI- D-09-00086.
24. Ramaglia L, Postiglione L, Di Spigna G, Capece G, Salzano S, Rossi G. Sandblasted-acid-etched titanium surface influences in vitro the biological behavior of SaOS-2 human osteoblast-like cells. Dent Mater J. 30(2):183-92. doi: 10.4012/dmj.2010-107.
25. Zareidoost A, Yousefpour M, Ghaseme B, Amanzadeh A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J Mater Sci Mater Med. 2012;23(6):1479-88. doi: 10.1007/s10856-012-4611-9.
26. Annunziata M, Oliva A, Basile MA, Giordano M, Mazzola N, Rizzo A, et al. The effects of titanium nitride-coating on the topographic and biological features of TPS implant surfaces. J Dent. 2011;39(11):720-8. doi: 10.1016/j.jdent.2011.08.003.
27. Abron A, Hopfensperger M, Thompson J, Cooper LF. Evaluation of a predictive model for implant surface topography effects on early osseointegration in the rat tibia model. J Prosthet Dent. 2001;85(1):40-6.
28. Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J J Biomed Mater Res. 1998;40(1):1- 11. doi: 10.1002/(SICI)1097-4636(199804)40:1<1::AIDJBM1> 3.0.CO;2-Q.
29. Park JY, Davies JE. Red blood cell and platelet interactions with titanium implant surfaces. Clin Oral Implants Res. 2000;11(6):530- 9. doi: 10.1034/j.1600-0501.2000.011006530.x.
 Endereço para correspondência:
Endereço para correspondência:
GENNARO
e-mail: gfassis@fob.usp.br
Received on: 27/6/2011
Final version resubmitted on: 24/4/2012
Approved on: 31/8/2012













