Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.2 Porto Alegre Abr./Jun. 2013
REVISÃO / REVIEW
Stem cells and their niches: importance in tissue engineering applied to dentistry
Células-tronco e seus nichos: importância na engenharia de tecidos aplicada à odontologia
Cíntia de Vasconcellos MACHADOI; Ivana Lúcia Oliveira NASCIMENTOI; Paloma Dias da Silva TELLESII
I Universidade Federal da Bahia, Instituto de Ciências da Saúde, Departamento de Bio-Interação. Avenida Reitor Miguel Calmon, s/n., Vale do Canela, 40110- 100, Salvador, BA, Brasil.
II Universidade Federal da Bahia, Faculdade de Odontologia, Departamento de Odontologia Social e Pediátrica. Salvador, BA, Brasil.
ABSTRACT
Niches are special microenvironments in tissue where stem cells are located. At these sites, which are a compound of stromal cells, extracellular matrix and soluble factors, complex molecular interactions that maintain the essential properties of stem cells occur, such as self-renewal and differentiation into multiple lineages, according to the organism's needs. Some adult stem cell niches have already been described, but the majority of them remain unclear, including the dental pulp stem cell niches. Dental pulp stem cells have been isolated from deciduous and permanent teeth and have the potential to self-renew and differentiate. However, little is known about the exact anatomic location of these cells, and the relationship between stem cells and surrounding cells in dental pulp. Understanding how stem cells behave in the niche is extremely important in order to extract these cells from their natural habitat, expand them in vitro and transplant the stem cells back to the patient, to repair and/or regenerate tissues and organs, with no risks to the individual's integrity. Likewise, the knowledge of stem cell biology is crucial to the development of stem cell therapies, based on tissue engineering applied to dentistry, seeking the regeneration of dental tissues damaged or lost by caries, trauma or genetic diseases.
Indexing terms: Dental pulp. Stem cells. Stem cell niche.
RESUMO
Os nichos são microambientes especiais nos tecidos onde células-tronco de várias origens estão localizadas. Nestes sítios específicos, formados por vários tipos de células, matriz extracelular e fatores solúveis, complexas interações moleculares ocorrem para que a célulatronco mantenha sua capacidade de autorrenovação e permaneça no seu estado indiferenciado ou se especialize em determinada linhagem celular, atendendo desta maneira as necessidades do organismo. Alguns nichos de células-tronco adultas já foram descritos, embora a maioria permaneça desconhecida, como o das células-tronco pulpares. As células-tronco pulpares, já foram isoladas tanto de dentes decíduos como de permanentes e apresentam as características essenciais de uma célula-tronco, como capacidade de autorrenovação e multi-diferenciação. Apesar disso, pouco se sabe a respeito da localização anatômica destas células na polpa, assim como as possíveis interações funcionais entre as células-tronco pulpares e as células do estroma circundante. O entendimento de como as células-tronco interagem com o microambiente onde estão inseridas é essencial para que se possa extrair as mesmas do seu habitat natural, cultivá-las in vitro e aplicá-las em diferentes sítios para que promovam o reparo e/ou regeneração de tecidos e órgãos, sem que isso represente um risco à integridade do organismo. Da mesma forma, o conhecimento de como estas células se comportam e respondem ao meio é fundamental para o desenvolvimento de terapias baseadas na utilização de células-tronco, que através da engenharia de tecidos aplicada à odontologia, visa à reestruturação de tecidos dentários danificados e/ou perdidos por cárie, trauma ou distúrbios genéticos.
Termos de indexação: Polpa dentária. Células-tronco. Nicho de células-tronco.
INTRODUCTION
Therapies based on the application of stem cells have great potential in the prevention and treatment of several diseases, such as cancer, diabetes, cardiovascular disease, spinal cord injuries, neurological diseases such as Parkinson's and Alzheimer's, and in the regeneration of various tissues and organs. However, further studies are required to gain complete understanding of stem cell biology, which is fundamental for the development of successful cell-based therapies1-3.
Stem cells are undifferentiated cells with an extraordinary capacity of self-renewal; that is, they have the ability to generate other stem cells and perpetuate themselves. Likewise, these cells give rise to progenitor cells committed to a particular cell lineage, and play a crucial role in tissue repair and homeostasis.
themselves. Likewise, these cells give rise to progenitor cells committed to a particular cell lineage, and play a crucial role in tissue repair and homeostasis.
According to the origin of these cells, they can be classified into embryonic stem cells (ESC) and adult stem cells (ASC). ESCs are pluripotent cells derived from the inner cell mass of the embryo at the blastocyst stage, and are able give rise to all three embryonic germ layers - ectoderm, endoderm, and mesoderm. However, these cells can induce the development of teratomas4-5. In addition, legal and ethical issues make it difficult to use these cells in scientific studies. On the other hand, ASCs are present in virtually all tissues and organs of an organism at different stages of development, and are able to differentiate into one or more cellular types, but not into all, such as ESCs. ASCs are also classified in hematopoietic stem cells (HSC) and mesenchymal stem cells (MSC), as shown in Figure 14,6-7.
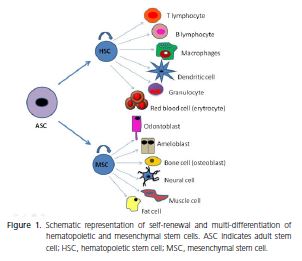
MSCs are considered one of the most promising stem cell types, due to their availability in tissues, multidifferentiation capacity, lack of ethical problems and do not form teratomas8. Nevertheless, until now, there has been no specific marker or combination of markers that identify MSCs9. For this reason, the isolation of MSCs also depends on the biological characteristics, such as colonyforming capacity, fibroblast-like morphology (these cells are also known as colony-forming unit fibroblasts - CFUFs), plastic adherence, vigorous proliferative ability, selfrenewal and multi-differentiation capacity8-9. Table 1 shows the surface markers frequently expressed by MSCs isolated from different tissues. Likewise, markers with little or no expression are listed10-17. 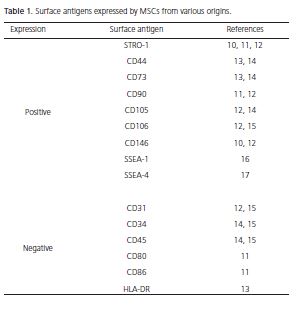
The key for the use of stem cell therapy in tissue and organ regeneration is the ability of SCs to differentiate into several cell types, depending on the stimulus received18. Stem cells present an enormous plasticity, being able to respond to the environment in which they are inserted, altering their original features and acquiring the characteristics of a given tissue, even if they had originated in a different site6. This ability of transdifferentiation has been evidenced in several studies19-21, emphasizing that the niche or microenvironment where the stem cells reside exerts a great influence on them22.
Stem cells: symmetric and asymmetric divisions
Most cells, including stem cells, can divide symmetrically, giving rise to two identical cells. However, stem cells also have the ability to undergo asymmetric divisions, in which two distinct daughter cells are generated. This process results in the generation of a stem cell daughter that remains in the niche and a progenitor daughter that leaves the niche and differentiates into a particular mature cell. Asymmetric division consists of a fundamental stem cell strategy to keep their self-renewal and differentiation capacity. An appropriate number of stem cells must be retained in the niche, and there must be a demand for specialized cells in the surrounding tissues, in order to maintain the organism homeostasis6,22-23.
What are the stem cells niches?
Stem cells are located in special microenvironments called niches, which protect these cells from damage, and from inappropriate differentiation or apoptotic stimuli, and other stimuli that could challenge stem cell reserves and compromise tissue homeostasis24. The niches are composed not only of stem cells, but also a diversity of differentiated cells, extracellular matrix and soluble factors. Complex interactions among the components of the niche allow stem cells to preserve their unique intrinsic properties for long periods, including the maintenance of their undifferentiated state, their self-renewal ability and capacity to give rise to different cell types22,25.
There is a constant cross-talk between stem cells and the surrounding niche cells that sometimes stimulates the differentiation of stem cells into progenitor cells, and sometimes protects them from many different stimuli. The primary function of the niche is to anchor stem cells and keep physical organization in a particular location in tissues. Adhesion molecules such as N-cadherins and integrins are essential for the maintenance of HSCs in the niche, for example. The microenvironment also exerts a regulatory function over stem cells, safeguarding excessive stem cell production that could lead to cancer23-24.
Thus, a hallmark of a functional niche is to maintain the perfect balance between quiescence and activity of stem cells. This delicate equilibrium plays a key role during embryonic development, as well as in tissue regeneration, replenishing lost cells due to apoptosis or due to tissue damage22-24. This special and unique relationship between stem cells and their niche occurs through direct cell-tocell contact and through the release of different soluble molecules, such as cytokines, chemokines and growth factors. The combination among the intrinsic genetic characteristics of stem cells and their microenvironment will drive their properties, as well as define the potential for the clinical application of these cells26.
Structure of different stem cell niches
The stem cell niches vary in nature and location depending on the tissue type23. Exactly when and how these stem cell sanctuaries" develop in tissues remains unclear22. What is known is that there is a considerable variation in structure and organization of these special microenvironments that shelter different types of stem cells27.
In mammals, some adult stem cell niches have already been identified successfully. Epithelial stem cells reside in the bulge area of hair follicles, near the sebaceous glands. Upon activation, these cells give rise to daughter cells that are retained in the bulge and remain as stem cells, or to progenitor cells responsible for hair regeneration. These cells can also convert to epidermal progenitors and replenish lost or damaged epidermis23,28. On the other hand, the intestinal stem cell niche was identified near the crypt base of the small intestine. In this region, the intestinal stem cells are in close contact and interaction with MSCs29.
Bone marrow hematopoietic stem cells (HSCs) are the best characterized stem cell population up until now24. HSCs are located proximal to the endosteal surface of trabecular bone in bone marrow, in direct contact with the osteoblasts. HSCs are attached to osteoblasts through a specific adhesive interaction between N-cadherin and b-catenin30, although other adhesion molecules, such as integrins22, are important in this process. In this location, MSCs appear to be an important component of the HSC niche31. HSCs were also identified in association with blood vessels in bone marrow, indicating that more than one niche may harbor stem cells in the same tissue32.
In the nervous system, the neural stem cells (NSC) are located near the blood vessels in the subventricular zone of the lateral ventricle and in the subgranular zone of the hippocampus region33. The endothelial cells, which are essential components of the NSC niche, provide the adhesion of the stem cells in these sites and generate a variety of signals that control their self-renewal and lineage differentiation34.
Stem cells in the dental pulp and the niche
Mesenchymal stem cells can be isolated from almost all tissues in the organism, including dental pulp. Although mesenchymal stem cells have already been isolated from deciduous and permanent teeth, there is a lack of information regarding the precise anatomical location of these cells19,35-36. This is mainly attributed to the rarity of stem cells in the pulp, as well as the absence of specific MSC markers that identify different developmental stages of these cells during odontogenesis, such as ectomesenchymal stem cells, cells from dental papilla, dental pulp stem cells, precursor cells from the pulp, preodontoblasts and mature odontoblasts9-10.
In a traditional view of dental pulp, the cell rich zone situated close to the odontoblast layer shelters a population of stem/progenitors cells, serving as a reservoir for the replacement of odontoblasts damaged by carious processes9. However, Shi & Gronthos10 demonstrated that the location of stem cells in dental pulp is restricted to the perivascular region and to the perineurium of dental pulp fiber nerves, but is absent in the odontoblastic layer and in the surrounding fibrous tissue. Likewise, it has been demonstrated that the damage caused to the dental pulp tissue stimulated the migration of stem/progenitor cells located in perivascular areas in dental pulp towards the injury site37.
According to Scadden25, both endothelial cells, pericytes such as the smooth muscle cells surrounding blood vessels may constitute the MSC niche, and contribute to a perivascular location of these cells. Recent studies that associated the location of MSCs with blood vessels, have suggested a strong correlation between MSCs and pericytes38. Typical pericyte markers, such as CD146, have also been expressed in MSCs from several tissues, including dental pulp10,39.
Location of MSCs at perivascular sites throughout the body would provide these cells with easy access to all tissues in the organism. In case of an injury, MSCs would be released by the rupture of blood vessels, migrate to the affected site and differentiate into the required cell type, promoting tissue repair40. In the affected area, MSCs would be capable of secreting immunomodulatory molecules, minimizing the extent of tissue damage and decreasing the inflammatory response, allowing tissue regeneration8,14. Similarly, the secretion of trophic factors by MSCs in the damaged area could inhibit apoptosis, stimulate angiogenesis, and stimulate the mitosis of tissueintrinsic progenitors41.
Importance of stem cells in dental pulp
Despite the technological advances in dentistry, to this date, no restorative material has been able to contemplate all the ideal physical, mechanical and biological properties to replace dental tissues4. Theoretically, biomaterials developed from autogenous tissues should be the best choice for clinical reconstruction of teeth lost or damaged by oral diseases, trauma or genetic disorders, in addition to the repair of craniofacial bone deffects9,42-44. Thus, tissue engineering applied to dentistry through the use of stem cell therapies, could contemplate this innovative and promising proposal in a masterful way.
MSCs obtained from the pulp of deciduous (SHEDs - stem cells from human exfoliated deciduous teeth)36 and permanent teeth (DPSCs - postnatal human dental pulp stem cells)35 may play a crucial role in the regeneration of the pulp-dentin complex, through their differentiation into functional odontoblasts. Different studies have shown that when these cells were transplanted into the subcutaneous space of immunocompromised mice, in association with biodegradable scaffolds and specific growth factors, areas of vascularized pulp tissue, surrounded by a layer of odontoblasts associated with dentin-like structures were observed3,19,35-36,45. According to Murray & Garcia-Godoy43, dental tissues developed from stem cells derived from human dental pulp have the same chemical, physical and esthetic characteristics as a natural tooth.
An efficient vascular network is fundamental for the correct functioning of a regenerated or implanted tissue, promoting an adequate supply of oxygen and nutrients. Recent studies have demonstrated the the potential of SHEDs to differentiate into vascular endothelial cells, induced by the presence of VEGF (vascular endothelial growth factor), which is considered the most important growth factor related to angiogenesis and vaculogenesis45-46. These differentiated endothelial cells were able to form functional blood vessels, which are essential for the development of a new tissue" in cases of pulp necrosis, for example. Likewise, dental pulp stem cells were capable of differentiating into osteoblasts, indicating that the use of stem cells may be a feasible therapy in cases of several bone loss due to periodontal disease, trauma or anodontia47.
Mesenchymal stem cells isolated from dental pulp can be considered a promising alternative in the treatment of various conditions, such as muscular dystrophy, spinal cord injuries, autoimmune diseases, ischemic disorders, among others, in addition to the regeneration of orofacial structures, emphasizing the ability of DPSCs and SHEDs to specialize into different cell types45,48-50. The combination of characteristics such as self-renewal, high proliferation capacity, as well as the easy access to them and their availability, make the dental pulp an attractive source of MSCs for tissue regeneration, especially those cells extracted from deciduous teeth, which are usually disposed of after physiological exfoliation3.
FINAL CONSIDERATIONS
The knowledge of how stem cells are inserted in their physiological microenvironment is crucial for the elucidation of the biology of these cells. In other words, understanding how niche cells and the extracellular matrix control the fate of stem cells is critical for the development of therapies that apply stem cells in the prevention and treatment of several diseases, as well as in the regeneration of organs and tissues such as teeth and craniofacial structures.
Collaborators
All authors participated in the conception, data collection and composition of the article.
REFERENCES
1. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9-20. doi: 10.1161/01.RES.0000135902.99383.6f.12. Newton AV. Denture sore mouth: a possible ætiology. Br Dent J. 1962;102(1):357-60. [ Links ]
2. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33(4):377-90. doi: 10.1016/j.joen.2006.09.013.
3. Casagrande L, Cordeiro MM, Nör SA, Nör JE. Dental pulp stem cells in regenerative dentistry. Odontology. 2011;99(1):1-7. doi: 10.1007/s10266-010-0154-z.
4. Nadig RR. Stem cell therapy: hype or hope? A review. J Conserv Dent. 2009;12(4):131-8. doi: 10.4103/0972-0707.58329.
5. Asano T, Sasaki K, Kitano Y, Terao K, Hanazono Y. In vivo tumor formation from primate embryonic stem cells. Methods Mol Biol. 2006;329:459-67.
6. Cai J, Weiss ML, Rao MS. In search of stemness". Exp Hematol. 2004;32(7):585-98. doi: 10.1016/j.exphem.2004.03.013.
7. Da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204-13. doi: 10.1242/jcs.02932.
8. Da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287- 99. doi: 10.1634/stemcells.2007-1122.
9. Yan M, Yu Y, Zhang G, Tang C, Yu J. A journey from dental pulp stem cells to a bio-tooth. Stem Cell Rev Rep. 2011;7(1):161-71. doi: 10.1007/s12015-010-9155-0.
10. Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696-704. doi: 10.1359/jbmr.2003.18.4.696.
11. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726-36. doi: 10.1038/ nri2395.
12. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble Jm, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99(5):1285-97. doi: 10.1002/jcb.20904.
13. Govindasamy V, Ronald VS, Totey S, Din SB, Mustafa WM, Totey S, et al. Micromanipulation of culture niche permits longterm expansion of dental pulp stem cells - and economic and commercial angle. In Vitro Cell Dev Biol Anim. 2010;46(9):764- 73. doi: doi: 10.1007/s11626-010-9332-0. Collaborators All authors participated in the conception, data collection and composition of the article.
14. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815-22. doi: 10.1182/blood-2004-04-1559.
15. Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181(1):67-73. doi: 10.1002/(SICI)1097- 4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C.
16. Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109(1):1298-306. doi: 10.1182/blood-2006-06-030551.
17. Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RCR. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(15):1743-51. doi: 10.1182/ blood-2005-11-010504.
18. Kolya CL, Castanho FL. Células-tronco e a odontologia. ConScientiae Saúde. 2007;6 (1):165-71.
19. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531-5. doi: 10.1177/154405910208100806.
20. Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M, et al. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, addipogenesis, and neurogenesis. Stem cells. 2006;24(11):2493-503. doi: 10.1634/stemcells.2006-0161.
21. Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787-95. doi: 10.1634/stemcells.2007-0979.
22. Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(19):769-78. doi: 10.1016/S0092-8674(04)00255-7.
23. Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605-31.
24. Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880-5. doi: 10.1126/science.1110542.
25. Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075-9. doi:10.1038/nature04957. 16. 21. Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos
26. Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70 (23):9969-78. doi: 10.1158/0008-5472.CAN-10-1712.
27. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598-611. doi: 10.1016/j.cell.2008.01.038.
28. Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351-4. doi:10.1038/nm1328.
29. Booth C, Potten C. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105(11):1493-9. doi:10.1172/ JCI10229.
30. Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836-41. doi:10.1038/ nature02041.
31. Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313(16):3377- 85. doi: 10.1016/j.yexcr.2007.07.027.
32. Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish haematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109-21. doi: 10.1016/j.cell.2005.05.026.
33. Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13(5):543-50. doi: 10.1016/j.gde.2003.08.012.
34. Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338-40. doi: 10.1126/science.1095505.
35. Gronthos S, Mankani M, Brahim J, Robey G, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. PNAS. 2000;97(25):13625-30.
36. Miura M, Gronthos S, Zhao M, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. PNAS. 2003;100(10):5807-12. doi: 10.1073/pnas.0937635100.
37. Téclès O, Laurent P, Zygouritsas S, Burger AS, Camps J, Dejou J, et al. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol. 2005;50(2):103- 8. doi: 10.1016/j.archoralbio.2004.11.009.
38. Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;11(3):301-13. doi: 10.1016/j.stem.2008.07.003.
39. Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;11(3):229- 30. doi: 10.1016/j.stem.2008.08.008.
40. Kolf CM, Cho E, Tuan RS. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204.
41. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076-84. doi: 10.1002/ jcb.20886.
42. Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83(7):523-8. doi: 10.1177/154405910408300703.
43. Murray PE, Garcia-Godoy F. Stem cell responses in tooth regeneration. Stem Cells Dev. 2004;13(3):255-62. doi:10.1089/154732804323099181.
44. Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428-34.
45. Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells form exfoliated deciduous teeth. J Endod. 2008;34(8):962-9. doi: 10.1016/j.joen.2008.04.009.
46. Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MAAM, Shi S, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89(8):791-6. doi: doi: 10.1177/0022034510368647.
47. d'Aquino R, Graziano A, Sampaolesi M, Laino G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162- 71. doi:10.1038/sj.cdd.4402121.
48. Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238(1):120-32. doi: 10.1006/dbio.2001.0400.
49. Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Zucconi E, Fonseca SA, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J Transl Med. 2008;6:35. doi: 10.1186/1479-5876-6-35.
50. Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1(1):5. doi: 10.1186/scrt5.
 Endereço para correspondência:
Endereço para correspondência:
CV MACHADO
e-mail: cintiamachado@hotmail.com
Received on: 22/3/2011
Final version resubmitted on: 5/5/2011
Approved on: 23/9/2011













