Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.3 Porto Alegre Jul./Set. 2013
ORIGINAL / ORIGINAL
Antimicrobial activity of three temporary endodontic coronal sealers
Atividade antimicrobiana de três seladores coronários temporários em Endodontia
Flávio Rodrigues Ferreira ALVESI; Bianca Poncioni Santos GUILHERMEI; Tatiana Vasconcellos FONTESI; Mariana Pires CRESPOI; Jardel Camilo do Carmo MONTEIROI; Julio Cezar Machado de OLIVEIRAI
I Universidade Estácio de Sá, Faculdade de Odontologia, Departamento de Endodontia. Av. Alfredo Baltazar da Silveira, 580, Recreio, 22790-710, Rio de Janeiro, RJ, Brasil.
ABSTRACT
Objective
The object of the present study is to analyze, in vitro, the antimicrobial activity of three temporary endodontic coronal sealers.
Methods
The materials tested were Tempo® (Vigodent, Rio de Janeiro, Brazil), IRM® (Dentsply, Petrópolis, Brazil) and Coltosol® (Vigodent, Rio de Janeiro, Brazil). The agar diffusion method was used for this analysis. Nine plates containing the agar blood culture medium were inoculated with human saliva and in each plate three equidistant cavities were made and filled with one of the materials tested. Two plates were not inoculated and served as the negative control of the culture medium. All the plates were incubated in bacteriological incubators, in aerobiosis, for 48 hours, at 37oC. The inhibition halos of bacterial growth were measured in millimeters.
Results
Tempo® (Vigodent, Rio de Janeiro, Brazil) did not show a inhibition halo of bacterial growth in any of the nine plates. Coltosol® (Vigodent, Rio de Janeiro, Brazil) produced halos in all plates, and IRM® (Dentsply, Petrópolis, Brazil) in 4 out of 9 plates. In all the tests, the halos produced by Coltosol® (Vigodent, Rio de Janeiro, Brazil) were more pronounced than the ones produced by IRM® (Dentsply, Petrópolis, Brazil) (p<0.05). The temporary coronal sealer Coltosol® (Vigodent, Rio de Janeiro, Brazil) presented the most prominent antimicrobial activity, followed by the temporary coronal sealer IRM® (Dentsply, Petrópolis, Brazil).
Conclusion
Among the materials tested, it was concluded that the Coltosol® (Vigodent, Rio de Janeiro, Brazil) presented the highest antimicrobial activity.
Indexing terms: Dental restoration temporary. Endodontics. Products with antimicrobial action.
RESUMO
Objetivo
Avaliar, in vitro, a atividade antimicrobiana de três seladores coronários temporários utilizados em Endodontia.
Métodos
Os materiais testados foram o Tempo® (Vigodent, Rio de Janeiro, Brasil), IRM® (Dentsply, Petrópolis, Brasil) e Coltosol® (Vigodent, Rio de Janeiro, Brasil). O método utilizado para a avaliação foi o teste de difusão em ágar. Nove placas contendo o meio de cultura ágar-sangue foram inoculadas com saliva humana e em cada uma foram confeccionados 3 furos eqüidistantes sendo cada um preenchido com um dos materiais testados. Duas placas foram inoculadas e serviram como controle negativo do meio de cultura. Todas as placas foram incubadas em estufa bacteriológica, em aerobiose, por 48 horas, à temperatura de 37ºC. Os halos de inibição do crescimento bacteriano foram medidos em milímetros.
Resultados
O selador temporário Tempo® (Vigodent, Rio de Janeiro, Brasil) não apresentou halo de inibição do crescimento bacteriano nas nove placas. Coltosol® (Vigodent, Rio de Janeiro, Brasil) produziu halo de inibição em todas as placas enquanto que o IRM® (Dentsply, Petrópolis, Brasil) produziu halo em 4 das 9 placas. Em todos os testes, os halos produzidos pelo Coltosol® (Vigodent, Rio de Janeiro, Brasil) foram mais pronunciados que os do IRM® (Dentsply, Petrópolis, Brasil) (p<0.05). O selador coronário temporário Coltosol® (Vigodent, Rio de Janeiro, Brasil) apresentou a atividade antimicrobiana mais pronunciada, seguido pelo IRM® (Dentsply, Petrópolis, Brasil). O selador temporário Tempo® (Vigodent, Rio de Janeiro, Brasil) não apresentou qualquer atividade antimicrobiana.
Conclusão
Dentre os materiais testados, podemos concluir que o Coltosol® (Vigodent, Rio de Janeiro, Brasil) é o que apresenta maior atividade antimicrobiana.
Termos de indexação: Restauração dentária temporária. Endodontia. Produtos com ação antimicrobiana.
INTRODUCTION
The literature is controversial about the role of coronal sealing in endodontic treatment success. For some authors, the coronal seal of root canals is as important as the apical seal1-4, but a recent prospective study with 1369 cases, regularly followed for a five-year period, found that the quality of the coronal restoration does not affect treatment outcome5. Although the discussion about the importance of permanent coronal sealing still remain, the importance of temporary coronal sealing is unquestionable. The main objective of the temporary restoration is to prevent the contamination of the root canals by food, fluids from the oral cavity and microorganisms6. During the endodontic treatment, the conditions of cleanliness which are achieved after the preparation of the root canals can only be assured by the use of temporary restorative materials which prevent microbial ingress7. Amongst the desirable properties of such materials, these can be highlighted: sealing ability, biocompatibility, dimensional stability, ease of manipulation, insertion and removal, low cost, low solubility and antimicrobial activity. The antimicrobial activity is essential to avoid contamination of the root canals between appointments or after treatment before the permanent restoration.
The endodontic literature contains some studies of the antimicrobial activity of the temporary sealing materials. Siqueira et al.8, tested the antimicrobial activity of six materials used as temporary seals in endodontics [Coltosol® (Vigodent, Rio de Janeiro, Brazil), Pulpo-San® (S.S. White, Rio de Janeiro, Brazil), Zinc-Oxide and Eugenol cement - ZOE® (Dentsply, Petrópolis, Brazil), Intermediate Restaurative Material - IRM® (Dentsply, Petrópolis, Brazil), Durelon (3M Espe, Minnesota, United States of American) and Glass Ionomer cement - Vidrion R® (S.S. White, Rio de Janeiro, Brazil)] using the agar diffusion test, in which Streptococcus bacteria were seeded and the materials tested were inserted. All the materials tested showed antimicrobial activity - Coltosol® (Vigodent, Rio de Janeiro, Brazil), Pulpo-San® (S.S. White, Rio de Janeiro, Brazil) and ZOE® (Dentsply, Petrópolis, Brazil) were the most efficient and Vidrion-R® (S.S. White, Rio de Janeiro, Brazil) the least efficient, producing the smallest inhibition halos of bacterial growth. Kopper et al.9, used the agar diffusion test with mixed cultures of Enterococcus faecalis, Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans to test the antimicrobial activity of 10 temporary sealing materials. Results showed that none of the temporary sealing materials tested presented antimicrobial activity under anaerobiotic conditions. Slutzky et al.10 investigated the antibacterial properties of Revoltek LC® a composite resin (GC America Inc, Alsip, United States of American), Tempit® a zinc oxide material (Centrix, Shelton, United States of American), Systemp inlay® a composite resin (Ivoclar Vivadente LTDA, Barueri, Brazil), and IRM® (Dentsply, Petrópolis, Brazil) by the direct contact test with Streptococcus mutans and Enterococcus faecalis. The materials were examined immediately after setting, 1, 7, 14, and 30 days. Systemp inlay® (Ivoclar Vivadente LTDA, Barueri, Brazil), Tempit® (Centrix, Shelton, United States of American), and IRM® (Dentsply, Petrópolis, Brazil) exhibited antibacterial properties when in contact with S. mutans for at least 7 days, Tempit® (Centrix, Shelton, United States of American) and IRM® (Dentsply, Petrópolis, Brazil) sustained this ability for at least 14 days. When in contact with E. faecalis Tempit® (Centrix, Shelton, United States of American) and IRM® (Dentsply, Petrópolis, Brazil) were antibacterial immediately after setting, IRM® (Dentsply, Petrópolis, Brazil) sustained this ability for at least 1 day.
As follows, we present the composition of the coronal sealers used in this study. Intermediate Restorative Material - IRM® (Dentsply, Petrópolis, Brazil) is a Zinc- Oxide and Eugenol cement reinforced with polymethyl methacrylate. The IRM® (Dentsply, Petrópolis, Brazil) material powder is composed of 20% zinc-oxide, 80% polimetil- metacrilato and the IRM® (Dentsply, Petrópolis, Brazil) material liquid is composed of 99,5% eugenol and 0,5% acetic acid. Coltosol® (Vigodent, Rio de Janeiro, Brazil) is another widely used temporary sealer which is composed of zinc-oxide, zinc sulphate-1-hydrate, calcium sulphate - hemihydrates, diatomaceous earth, dibutyl phthalate and copolymer - polyvinyl chloride. Its antimicrobial activity is attributed to the zinc ions which dissociate from the zincoxide and the zinc sulphate11. The last temporary sealer tested was Tempo® (Vigodent, Rio de Janeiro, Brazil). It is a photopolymerizable temporary sealing material whose composition consists of urethane difunctional acrylated, tegma, inhibitors, photoinitiators and highly dispersed signalized silicon dioxide.
The object of the present in vitro study is to comparatively analyze the antimicrobial activity of the three above-mentioned temporary coronal sealing materials.
METHODS
The materials tested in the present study, Tempo® (Vigodent, Rio de Janeiro, Brazil), IRM® (Dentsply, Petrópolis, Brazil) and Coltosol® (Vigodent, Rio de Janeiro, Brazil) were purchased in dental supply stores in the city of Niteroi - RJ, Brazil (Figure 1). The lot codes and expiration dates on the boxes used were as follows: Coltosol® (Vigodent, Rio de Janeiro, Brazil), lot code 0174.925, expiration date 02/2012; IRM® (Dentsply, Petrópolis, Brazil), lot code 050.931-A, expiration date 08/2010; and Tempo® (Vigodent, Rio de Janeiro, Brazil), lot code 061/09, expiration date 05/2012. The saliva sample was collected in a sterilized universal collector and part of it was inoculated in a tube containing tryptic soy broth (Newprov, Brazil) and incubated in aerobiosis, at 37oC, for 48 hours.
The method used to analyze the antimicrobial activity was the agar diffusion test. Eleven Petri dishes (50 X 9mm) containing the sheep agar blood culture medium (Newprov, Brazil) were used. A portion of the saliva culture (0.1ml) was seeded on the culture medium and spread using a sterile swab. This procedure was utilized in 9 plates that formed the experimental group. The 2 remaining plates were used as the negative control of the culture medium.
Three equidistant points were marked on the back of each plate. Under strict aseptic conditions, utilizing sterilized same diameter test tubes, three cavities (corresponding to the three equidistant points previously marked) were made in the agar around the flame produced by a Bunsen burner.
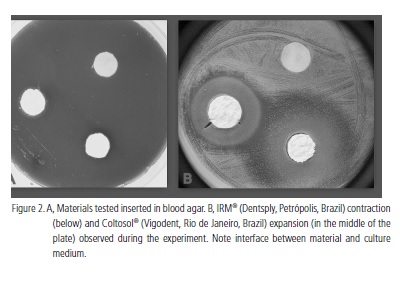
In each cavity, one of the materials was inserted. Therefore, the three materials were tested simultaneously and under the same conditions in each plate (Figure 2A). Each cavity was properly filled with one of the sealing materials utilizing a Centrix® syringe (Centrix, Shelton, United States of American) and a sterile spatula. All the materials were manipulated under aseptic conditions. The sealer IRM® (Dentsply, Petrópolis, Brazil) was prepared according to the manufacturer's recommendations. The sealers Tempo® (Vigodent, Rio de Janeiro, Brazil) and Coltosol® (Vigodent, Rio de Janeiro, Brazil) were inserted directly into the cavities, as they are ready to use and the sealer Tempo® (Vigodent, Rio de Janeiro, Brazil) was photopolimerized for 40 seconds. After the plates were prepared they were kept in a bacteriological incubator in aerobiosis for 48 hours at 37oC.
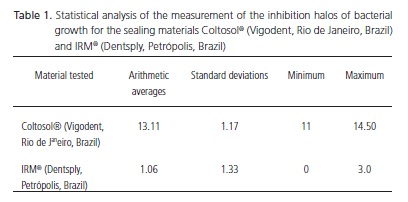
After that, the inhibition halos of bacterial growth were measured with a millimeter ruler by an examiner, through measuring the diameter of the halo, from one end to another of the circle, including the diameter of the cavities and the results were recorded (Table 1). The Mann- Whitney nonparametric test was used for the statistical analysis of the results.
The study was approved by Ethics Committee of the Estácio de Sá University (project nº 0137.0.308.000- 10, approval protocol nº 0155).

RESULTS
The temporary sealer Tempo® (Vigodent, Rio de Janeiro, Brazil) did not present inhibition halo of bacterial growth in any of the tests performed (n=9).
The temporary sealer Coltosol® (Vigodent, Rio de Janeiro, Brazil) produced inhibition halos of bacterial growth in all the tests performed, whereas temporary sealer IRM® (Dentsply, Petrópolis, Brazil) showed inhibition halos in 4 out of 9 tests performed. In all the tests performed, the halos produced by Coltosol® (Vigodent, Rio de Janeiro, Brazil) were more pronounced than the ones produced by IRM® (Dentsply, Petrópolis, Brazil). The average diameter of the halos produced by Coltosol® (Vigodent, Rio de Janeiro, Brazil) was 13mm, ranging from 11mm to 15mm, whereas the average diameter of the halos produced by IRM® (Dentsply, Petrópolis, Brazil) was 1,0mm, ranging from zero to 3mm. The arithmetic average, the standard deviation, the minimum and maximum values, as well as the statistical significance value are expressed on Table 1. The Mann-Whitney nonparametric test was applied to the results obtained by Coltosol® (Vigodent, Rio de Janeiro, Brazil) and by IRM® (Dentsply, Petrópolis, Brazil), and a significant difference (p=0,001) was found.
DISCUSSION
The endodontic literature contains few accounts of studies comparing the antimicrobial activity of temporary coronal sealers, which makes the comparison of the results of the present study difficult. The materials utilized in the present study were selected because of their market availability and widespread use amongst endodontics specialists.
In the agar diffusion test, the size of the inhibition halo of microbial growth depends on the solubility and diffusibility of the substance. Therefore, it cannot reach its full potential. However, in the root canal, these properties are also connected to the antimicrobial action in that environment12.
According to the results obtained in this study, the temporary sealer Tempo® (Vigodent, Rio de Janeiro, Brazil) did not show any antimicrobial activity. It is our belief that occurred due to the fact that after the photopolymerization, the material becomes inert. Nevertheless, Antunes et al.13, have analyzed the antimicrobial activity of photopolymerizable and non photopolymerizable glass ionomers manufactured by the same company, Vitremer® (3M, Sumaré, Brazil), and have observed that both produced significative inhibition halos of bacterial growth in a Streptococcus mutans culture. It should be pointed out, however, that the fluoride found in glass ionomers presents substantivity, which may be related to the antimicrobial activity even after photopolymerization.
The temporary coronal sealer Coltosol® (Vigodent, Rio de Janeiro, Brazil) presented significantly superior antimicrobial activity compared to IRM® (Dentsply, Petrópolis, Brazil). This result is in accordance with the study of Vágula et al.14. Although IRM® (Dentsply, Petrópolis, Brazil) contains eugenol, which is a recognized antimicrobial substance, its bacterial inhibition was inferior to the one observed with Coltosol® (Vigodent, Rio de Janeiro, Brazil). This may be due to the fact that the temporary sealer IRM® (Dentsply, Petrópolis, Brazil) is reinforced with an acrylic resin polymer which may interfere in its antimicrobial activity8. Another variable which may have influenced such results is the contraction observed with IRM® (Dentsply, Petrópolis, Brazil). After 48 hours in the bacteriological incubator, the temporary sealer IRM® (Dentsply, Petrópolis, Brazil) in each of the Petri dishes showed signs of contraction. Because of this reduction in volume, some areas of the material in each cavity in the agar were no longer in contact with the culture medium. On the other hand, the opposite was observed with temporary sealer Coltosol® (Vigodent, Rio de Janeiro, Brazil). In all tests performed with this temporary sealer, it expanded, causing the whole surface of the material to be in contact with the culture medium (Figure 2B). Such expansion may be a determinant factor in its superior antimicrobial activity. Barroso et al.15, having analyzed the dimensional stability of temporary sealers utilized in endodontics, came to the conclusion that Coltosol® (Vigodent, Rio de Janeiro, Brazil) showed an average expansion of 62% of its initial volume. According to Hosoya et al.16 and Uçtasli & Tinaz17, Coltosol® (Vigodent, Rio de Janeiro, Brazil) has a high degree of linear expansion which results from water absorption during its hardening process.
The results of the present study diverge from the results of the study performed by Kopper et al.9, who reported not having observed inhibition halos of bacterial growth in any of the 10 temporary sealers [including Coltosol® (Vigodent, Rio de Janeiro, Brazil)] tested by the same method utilized in the present study (agar diffusion). It must be pointed out that the absence of inhibition halos of bacterial growth in all the materials tested may indicate that an inadequate protocol might have been applied to differentiate the antimicrobial activity of the different materials tested in the above-mentioned study. Although IRM has presented less antimicrobial effect in comparison with Coltosol® (Vigodent, Rio de Janeiro, Brazil), Slutzky et al.10 found expressive antimicrobial effect of this material compared with two resin-based materials and another zinc oxide material. The time of antimicrobial activity of this material was also higher.
The choice of the agar blood culture medium was due to the fact that it is a culture medium that favors the growth and multiplication of a plethora of bacterial species. It is a rich, nonselective medium, more than adequate for the study of bacteria involved in human infections, mainly mesophilic bacteria, whose optimal growth happens at temperatures varying from 20oC to 45oC18. All the materials were tested under aerobiotic conditions, due the fact that the microorganisms in saliva are predominantly aerobic.
The results of the present study emphasize that when choosing a temporary coronal sealing material, not only should properties like dimensional stability, biocompatibility and solubility be taken into account, but also the antimicrobial activity, since this property can prevent the contamination or recontamination of the root canals after endodontic procedures and since the temporary coronal sealers available in the market differ greatly when it comes to their effective antimicrobial activity.
CONCLUSION
Under the conditions of the present in vitro study, it can be determined that amongst the materials tested, the temporary coronal sealer Coltosol® (Vigodent, Rio de Janeiro, Brazil) presented the most prominent antimicrobial activity, followed by the temporary coronal sealer IRM® (Dentsply, Petrópolis, Brazil). The temporary sealer Tempo® (Vigodent, Rio de Janeiro, Brazil) did not present any antimicrobial activity.
Collaborators
Idealization, study design and revision of the Portuguese version: FRF ALVES. Running the experiment: BPG SANTOS, TF VASCONCELLOS, MP CRESPO, JCC MONTEIRO and FRF ALVES. Statistical analysis: BPG SANTOS. Analysis of results and review of the literature: JCM OLIVEIRA.
REFERENCES
1. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995;28(1):12-8. [ Links ]
2. Sidaravicius B, Aleksejuniene J, Eriksen HM. Endodontic treatment and prevalence of apical periodontitis in an adult population of Vilnius, Lithuania. Endod Dent Traumatol. 1999;15(5):210-5. doi: 10.1046/j.1365-2591.2003.00640.x.
3. Dugas NN, Lawrence HP, Teplitsky PE, Pharoah MJ, Friedman S. Periapical health and treatment quality assessment of root filled teeth in two Canadian populations. Int Endod J. 2003;36(3):181- 92. doi: 10.1046/j.1365-2591.2003.00640.x.
4. Kirkevang LL, Orstavik D, Horsted-Bindslev P, Wenzel A. Periapical status and quality of root fillings and coronal restorations in a Danish population. Int Endod J. 2000;33(6):509-15. doi: 10.1046/j.1365-2591.2000.00381.x.
5. Ricucci D, Russo J, Rutberg M, Burleson JA, Spångberg LS. A prospective cohort study of endodontic treatments of 1,369 root canals: results after 5 years. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):825-42. doi: 10.1016/j. tripleo.2011.08.003.
6. Anderson RW, Powell B, Pashley D. Microleakage of three temporary endodontic restorations. J Endod. 1988;14(10):497- 501. doi: 10.1016/S0099-2399(88)80107-9.
7. Carvalho MGP. A importância dos materiais seladores temporários para o sucesso do tratamento integrado na clinica odontológica. Rev Dent On Line. 2004;3(9):15-8.
8. Siqueira Jr JF, Fraga RC, Lopes HP. Avaliação da atividade antibacteriana de materiais seladores temporários. JBC J Bras Clin Estét Odontol. 1999;3(15):67-9.
9. Kopper PMP, Andrade MLM, Só MVR, Oliveira EPM, Carvalho MGP, Bammann LL. Avaliação in vitro da atividade antimicrobiana de dez materiais seladores temporários livres de eugenol frente a uma cultura mista. JBE. 2002;3(8):28-32.
10. Slutzky H, Slutzky-Goldberg I, Weiss EI, Matalon S. Antibacterial properties of temporary filling materials. J Endod. 2006;32(3):214-7. doi: 10.1016/j.joen.2005.10.034.
11. Deveaux E, Hildelbert P, Neut C, Boniface B, Romond C. Bacterial microleakage of Cavit, IRM and Term. Oral Surg Oral Med and Oral Pathol. 1992;74(5):634-43.
12. Estrela C, Ribeiro RG, Estrela CRA, Pécora JD, Sousa-Neto MD. Antimicrobial effect of 2% sodium hypochlorite and 2% chlorherxidine tested by different methods. Braz Dent J. 2003;14(1):58-62. doi: 10.1590/S0103-64402003000100011.
13. Antunes ML, Silvestre FO, Beretta ALRZ, Imparato JCP, Pinheiro SL. Propriedades antimicrobianas de cimentos ionoméricos sobre cepas de S. mutans. Rev Assoc Paul Cir Dent. 2007;61(5):403-8.
14. Várgula MP, Pedott MM, Guimarães MRFSG, Aleixo RQ, Borré, MAM. Avaliação da ação antimicrobiana dos materiais seladores temporários utilizados pelos cirurgiões dentistas de ouro preto do oeste - RO. RESCO. 2010;1(1):21-30.
15. Barroso LS, Habitante SM, Gonçalves MIA. Análise da estabilidade dimensional de três materiais seladores provisórios utilizados em endodontia. JBE. 2001;2(7):278-82.
16. Hosoya NC, Cox CF, Arai T, Nakamura J. The walking bleach procedure: an in vitro study to measure microleakage of five temporary sealing agents. J Endod. 2000;26(12):716-8. doi: 10.1097/00004770-200012000-00011.
17. Uctasli MB, Tinaz AC. Microleakage of different types of temporary restorative materials used in endodontics. J Oral Sci. 2000;42(2):63-7.
18. Madigan MT, Martinko JM, Parker J. Microbiologia de Brock. São Paulo: Prentice Hall; 2004.
 Endereço para correspondência:
Endereço para correspondência:
FRF ALVES
e-mail: flaviofalves@uol.com.br
Received on: 30/12/2011
Final version resubmitted on: 23/1/2012
Approved on: 1/3/2012













