Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.4 Porto Alegre Out./Dez. 2013
REVISÃO / REVIEW
Microbiologic cross-contamination and infection control in intraoral conventional and digital radiology
Contaminação microbiológica e controle de infecção em radiologia intraoral convencional e digital
Luciana Maria Paes da Silva Ramos FERNANDESI; Ronald Ordinola ZAPATAII; Izabel Regina Fischer RUBIRA-BULLENI; Ana Lúcia Álvares CAPELOZZAI
I Universidade de São Paulo, Faculdade de Odontologia, Departamento de Estomatologia, Radiologia. Al. Octávio Pinheiro Brisola 9-75, Vila Universitária, 17012-901, Bauru, SP, Brasil.
II Universidade de São Paulo, Faculdade de Odontologia, Departamento de Endodontia. Bauru, SP, Brasil.
ABSTRACT
The microbiologic contamination can occur in all the steps of an intraoral conventional or digital radiographic examination, unless some measures to avoid the cross-infection are applied. Scientific articles have shown that during the radiograph taking procedure, there is contact with the patient's oral fluids (saliva and blood) and, consequently, the contamination of the film or digital sensor, film-holding devices, operator's hands and afterwards, the contamination of the surfaces, the X-Rays equipment, the processing environment and its solutions. Also, the literature shows that the existence of infection control protocols is mandatory for dental offices and colleges. The aim of this article is to review how the microbiologic contamination can occur in Dental Radiology practice and to describe more efficient methods to avoid it, in order to get a safe environment for patients, professionals and workers. It is extremely important that the professional be aware and use efficient protection barriers in all steps of an intraoral radiographic examination, whether conventional or digital.
Indexing terms: Dental clinics. Equipment contamination. Exposure to biological agents. Radiology.
RESUMO
A contaminação microbiológica pode ocorrer em todas as etapas de um exame radiográfico intra-oral convencional ou digital, caso não sejam tomadas medidas para evitar a infecção cruzada. Artigos científicos têm mostrado que durante a tomada radiográfica, existe o contato com fluidos orais do paciente (saliva e/ou sangue) e consequentemente, a contaminação de filmes radiográficos ou sensores digitais, posicionadores, mãos do operador e posteriormente, de superfícies do ambiente de trabalho, partes do aparelho de Raios-X, ambiente de processamento radiográfico e suas soluções. A literatura também mostra que a existência de protocolos de controle de infecção é obrigatória para consultórios e faculdades de Odontologia. Este trabalho se propõe a revisar como a contaminação microbiológica pode ocorrer na prática da Radiologia Odontológica e descrever os métodos mais efetivos para combatê-la, garantindo um ambiente seguro para pacientes, profissionais e funcionários que o freqüentam. É de fundamental importância que o profissional esteja consciente e utilize barreiras de proteção eficazes em todas as etapas do exame radiográfico intraoral, seja convencional ou digital.
Termos de indexação: Clínicas odontológicas. Contaminação de equipamentos. Exposição a agentes biológicos. Radiologia.
INTRODUCTION
The infection control currently plays a very important role in the practice of dentistry1. Since the beginning of the AIDS epidemic, an increased emphasis has been placed on medical and dental work practices2. Dental patients and dental health care workers are exposed to many infectious disease agents during the treatment, such as Mycobacterium tuberculosis, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Treponema pallidum and the viruses HIV, Hepatitis B, Hepatitis C, Herpes simplex 1 and 2, Cytomegalovirus, Epstein-Barr among others2-3. The cross-contamination is the passage of microorganisms from one person or object to another2. At the dental office, infections may be transmitted by direct contact (saliva, blood and other secretions) or indirect contact (saliva drips and contaminated aerosols). The development of infectious diseases occurs because of a lack of balance between the capacity of immunological defense of the host and the virulence of the pathogenic microorganism4.
Infection control practices are established to create and maintain a safe clinical environment to eliminate or minimize disease transmission during patient treatment2-3,5-6. Dental Radiology procedures are not invasive like Oral Surgery, Periodontology, Implantodology, for instance. However, the cross-contamination can occur since there is the contact with patient´ saliva and blood in dental radiology clinical routine5. For this reason, the protection with barriers before every radiographic taking procedure is important, in order to avoid the contact of contaminated operator's gloves with the X-Rays equipment and other high touch areas in the environment. The film handling after exposure can also be a source of contamination1. During routine use, dental radiographic film packets become contaminated with the patient's oral fluids and microflora5,8. The manipulation of the film packet during the processing of the radiography presents a potential for contamination of the darkroom equipment and its solutions8.
Digital radiography is becoming more common in dentistry, as it is considered to offer advantages such as reduced patient radiation exposure, elimination of film and darkroom equipment and ability to manipulate the images2-3,9-10. There are two basic types of receptors: direct sensors (CCD: charge-coupled device), which are attached to the computer monitor by a cable and indirect sensors (PSP: phosphor storage plate), which resemble intraoral film but are reusable and processed in a scanner2,9-12. Both of these receptors can become contaminated during exposure and manipulation. No receptor can be autoclaved and the use of effective barrier is recommended9-10,12-13.
The aim of the present article is to review how the cross-contamination can occur in intraoral dental radiology and to propose simple infection control protocols in order to avoid the contamination during the use of conventional or digital radiograph systems.
Contamination during the patient exposure to X-Rays
White & Glaze14, in 1978, found that the dental health care workers could transfer oral microorganisms from the patient's oral cavity to the radiographic equipment during routine intraoral radiography. It has also been found that Staphylococcus pyogenes could survive for at least 48 hours in the X-Rays equipment. Rahmatulla et al.15, in 1996, found that most high-touch areas in dental radiology, including the dental chair headrest adjustment lock, the X-Rays cone, the exposure control knob, the timer switch, the radiographic film placement area in the darkroom, the radiographic film feeding area in the automatic film processor and the revolving door to the darkroom became contaminated while taking radiographs. It has been concluded that, in order to eliminate the crosscontamination risk in intraoral radiology, the disinfection of the high-touch areas of the X-Rays equipment is mandatory.
Contamination of the intraoral dental film
Bajuscak et al.1, in 1993, used three types of papercovered and one type of plastic-covered Kodak dental film to determine if bacteria could penetrate the coverings and contaminate the inner film. The results indicate that papercovered films can be penetrated by bacteria and may go on to cause cross-contamination, whereas plastic-covered radiographic films provide an effective barrier to bacterial contamination.
Contamination during the dental film processing
Katz et al.16, in 1988, have shown that bacteria, when inoculated in very high concentrations, can survive in used dental radiography developer and fixer over a period of two weeks. Bachman et al.17, in 1990, measured bacterial contamination in a radiographic processing room during times of high and low clinical activity and processing effects on five types of microorganisms. The results show that contaminated films cross-contaminate radiographic equipment, and bacteria are not destroyed by the developing process. All bacteria tested survived processing on films and were found in the developer, fixer and water bath. The presence of these organisms indicates the need for infection control to prevent contamination. Stanczyk et al.8, in 1993, investigated microbiologic contamination of an automatic dental radiography processor and daylight loader during a week of simulated clinical use. Pure cultures of some microorganisms were used to contaminate intraoral radiographic film packets. During the opening of these packets, the end of each film was deliberately contaminated. These contaminated films and a group of non-contaminated films were then processed. The results revealed that the contamination of the processor and daylight loader occurred and remained even after 48 hours of inactivity. In addition, crosscontamination of the films occurred in the processor. In agreement with other researchers, this study has confirmed that the microorganisms can survive to the processing cycle and remain in the solutions and the parts of the processor and daylight loader.
Infection control during intraoral radiograph taking procedure
Katz et al.18, in 1989, made a survey concerning infection control practices in dental radiology to several U.S. and Canadian dental schools. The answers revealed that the majority of respondents have been following the infection control protocols suggested for Dental Radiology (such as surface disinfection, the use of disposable latex gloves, etc). However, some improvements were still necessary. The authors recommended the use of plastic coverage of the X-Rays equipment (cone, tubehead, control panel, exposure button) or an efficient disinfection of these surfaces before or after each patient. The infection control protocols must be followed not only for previously identified infectious patients. Silva et al.4, in 2004, evaluated the efficiency of an infection control protocol in Dental Radiology during the radiography taking procedure and processing. The suggested protocol presented some measures such as: the use of disposable gloves and overgloves during the radiograph taking procedure; the use of plastic barriers over the high-touch surfaces; the disinfection and sterilization of film-holding devices; the removal of contaminated overgloves in the darking room and film processing with new gloved hands. The researchers concluded that, after the establishment of the proposed infection control protocol, the number of microorganisms on the analyzed surfaces and processing solutions significantly reduced.
Infection control of intraoral dental film
Ash et al.19, in 1984, published an article that described a clinical aid devised to ensure aseptic radiographic technique in the treatment of patients with communicable disease. The authors recommended the use of a sealed plastic bag for radiographic dental film and its storage for ready access when needed. Sections of plastic bag are cut to a size larger than that of the dental film to be covered. The film is then sealed between the pieces of plastic using heat sealer. After the radiographic technique, the authors recommend the plastic bag removal and afterwards, the uncontaminated film processing. Ciola20, in 1988, published a readily adaptable, cost-effective method of infection control for dental radiography. The author suggested the covering of the dental film with finger cot before its placement on patient's mouth. As a result, the dental film does not have any contact with oral fluids and the cross-contamination is avoided. Neaverth and Pantera21, in 1991, suggested a disinfection protocol for dental films before their processing. The authors showed that the plastic-covered dental radiographic film can be disinfected by a 30-second immersion in 5.25% NaOCl, being effective, simple and suitable for routine use in Dental Radiology. Packota and Komiyama17, in 1992, undertook a study to determine the most effective method for the surface disinfection of saliva-contaminated dental radiographic film packets. The authors showed that mere physical removal of the saliva from the film packet, without the use of disinfectant, was not effective. They suggested and effective, practical and low cost protocol, which is the 30-seconds-immersion in NaOCl before processing in order to avoid cross-contamination. Pontual et al.7, in 2004, evaluated the effectiveness of the disinfection of intraoral saliva-contaminated dental film using the following methods: immersion during three different times (30 seconds, 2.5 and 5 minutes) and friction with 2% glutaraldehyde, 70% alcohol and 1, 2 and 5% sodium hypoclorite. Based on the results, the authors concluded that the immersion method was the most effective one, in 70% alcohol and 5% sodium hypoclorite solutions for at least 2.5 minutes.
Infection control in intraoral Dental Digital Radiology
Wenzel et al.18, in 1999, evaluated the efficacy of a simple cross-infection control procedure for CCD-based sensor and a storage phosphor plate after their use in a posterior bitewing examination. The CCD-based sensor was wrapped in a rubber tube (according to the manufacturer's instructions) and the phosphor plate was packed in the plastic envelope that comes with the system. After the exposure, the CCD-based sensor barrier was removed and disinfected with an alcohol tissue (70% ethanol and 2.5% chlorexidine digluconate solution), as well as its connection cable. The envelope with the phosphor plate was disinfected with the same solution and then it was cut open with a pair of scissors. Then, the authors used a pair of tweezers to pick the plate from the envelope without touching the outside part of the phosphor plate and to transfer the plate to the scanner. The research concluded that this simple and easy infection control protocol effectively avoided the cross contamination in dental digital radiology. Hokett et al.9, in 2000, published an article concerning the effectiveness of direct digital radiography barriers. The authors showed that the use of latex finger cots in conjunction with the standard plastic sheaths to cover the CCD sensor is a very effective procedure to prevent patient cross-contamination. Negron et al.11, in 2005, tested an infection control protocol for the PSP system. The results showed that the barrier envelopes used with the PSP sensors appeared to be an effective wayof reducing cross-contamination. Recently, Kalathingal et al.12 evaluated the contamination of PSP plates in a dental school. They noticed that the PSP plates are handled by a large number of people everyday and sometimes, not in a proper way, which can leads to the PSP microbiologic contamination. They conclude that meticulous infection control protocols are mandatory, as well as continuous training for staff and students. In a following research, Kalathingal et al.13 showed that the daily gas sterilization of PSP plates using ethylene oxide seems to be an effective solution, since the simple use of barriers are not enough to avoid the cross contamination of PSP systems in an environment like dental schools. Wenzel and Møystad10 highlighted the importance of hygiene precautions for the digital intraoral Radiology systems and stated that the digital sensors can be wiped with an alcohol-impregnated tissue. However, it is still unknown how much the sensors tolerate wiping.
DISCUSSION
The importance of infection control in Dental Radiology is highlighted in many scientific reports in literature1-23. During the clinical routine on a dental office, dental school or Radiology office, the X-Rays exposure to several patients is performed. Since there is contact with patient's oral fluids (saliva and blood), there is the possibility of microbiologic cross-contamination and the development of unexpected diseases from patient to patient, from patient to professional and dental workers and vice versa. Since the virulence or type of present bacteria are unknown, it is impossible to project the hazards for personnel who use the environment4,17. Besides, the identification of potentially infected patients through the clinical history, physical and clinical examinations are not always possible, then, the infection control protocols must be adopted for every patient4,18.
For this reason, the present article proposes simple and effective infection control procedures based on the literature review for every step of an intraoral radiographic examination, in order to assure aseptic and safe environment for patients, professionals and personnel.
The first step of the radiographic examination is the preparation of the environment and the exposure of the patient to the X-Rays. The protocol to be followed during the intraoral conventional exposure is: 1) Protect with barrier all the surfaces that can be touched by saliva contaminated gloves during exposure such as X-Rays equipment tubehead, cone, control panel, start button, chair and headrest adjustment controls and work area surfaces; 2) Prepare all the devices and radiographic films that will be used, properly cleaned and protected with plastic barrier (Figure 1); 3) Take the patient to the work area and place the lead apron and thyroid collar; 4) Wear gown, mask, eyewear and disposable new gloves in front of the patient; 5) Insert the radiographic film into the film holder device or directly in patient's mouth; 6) Execute the X-Rays exposure, avoiding touching any unprotected surface; 7) Remove the film holder device or radiographic film from patient's mouth and place the contaminated materials on a protected surface; 8) Remove contaminated gloves and wash hands; 9) Take the lead apron and thyroid collar off the patient; 10) Wear new gloves or overgloves and discard all contaminated protection barriers from the surfaces. Also, remove the film barrier or disinfect the film and film holder and if necessary, disinfect all surfaces that were not protected and could have had contact with oral fluids.

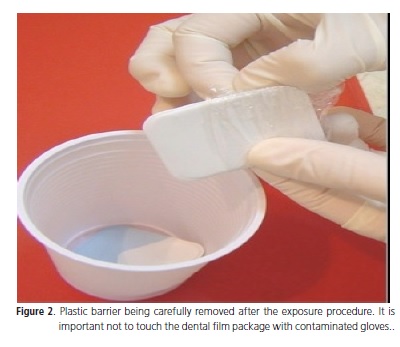
Considering the radiographic film, articles have shown that it can be a source of contamination, so, it must be protected with plastic barrier. If the barrier is not available, the dental film should be disinfected with alcohol 70%. The steps of the recommended protocol for the dental film handling is: 1) Protect every dental film with plastic barrier previously to its use; 2) Place carefully the protected film inside the patient's mouth wearing glove; 3) Take the patient to the work area and place the lead apron and thyroid collar; 4) After the exposure, take the dental film out of the patient's mouth and remove the plastic barrier, avoiding touching the dental film package (Figure 2); 5) Place the uncontaminated film inside a plastic cup (Figure 2); 6) Discard contaminated gloves and wash hands or wear overgloves; 7) Take the cup with uncontaminated films to the processing chamber.
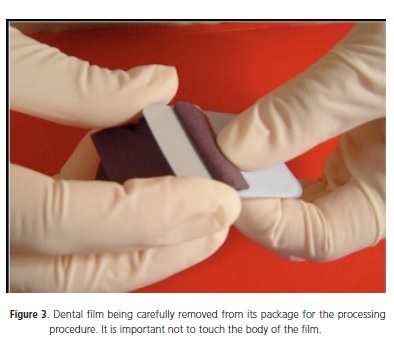
The radiographic processing is also a step of the conventional dental radiology routine, which can promote contamination of its solutions. The suggested protocol for this important step is: 1) Carefully open the package containing the radiographic film avoiding touching the body of the film with no gloves or wearing overgloves (Figure 3); 2) Place the film into the processor or inside developer recipient in case of a manual processing, by holding the film from its edges; 3) Discard film package in a proper recipient.
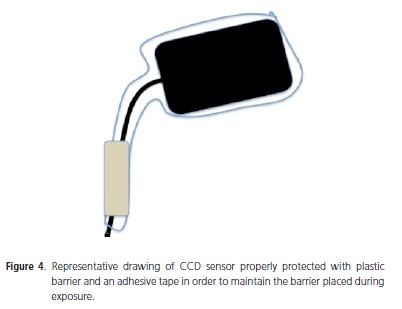
Concerning the new and advanced Digital Dental Radiology, the professional who uses direct or indirect systems must be aware about cross-contamination as well. Considering that CCD and PSP sensors are used many times in different patients, the use of protection barriers is mandatory. The suggested protocol for Digital Dental Radiology CCD (with sensor) system is: 1) Place plastic barrier which is able to protect the entire sensor and part of its cable (Figure 4); 2) Use rubber band or adhesive tape to maintain the barrier placed during exposure (Figure 4); 3) Place the sensor inside the patient's mouth carefully and proceed the exposure wearing gloves; 4) Take the protected sensor off the patient's mouth; 5) Carefully remove the plastic barrier without touching the sensor or its cable with contaminated gloves; 6) Disinfect the sensor and the cable with alcohol 70% solution.
The steps of the suggested protocol for Digital Dental Radiology PSP system is: 1) Place the protected PSP inside the patient's mouth wearing gloves and proceed the exposure; 2) Take the PSP from the patient's mouth; 3) Disinfect the plastic envelope with alcohol 70% solution; 4) Discard contaminated gloves or wear overgloves; 5) Carefully open the protection envelope with disinfected scissors and remove the PSP with tweezers, avoiding touching the body of the PSP; 6) Take the PSP to the scanner holding it by its edges; 7) Perform the gas sterilization using ethylene oxide of the PSP system following the manufacturer's instructions, at the end of a clinical day, in case of frequent use and handling by a large number of people (at dental schools or Radiology offices).
CONCLUSION
It is extremely important that the professional be aware and use efficient protection barriers in all steps of an intraoral radiographic examination, whether conventional or digital, in order to avoid the cross-contamination and the possibility of diseases to be developed.
Collaborators
LMPSR FERNANDES, RO ZAPATA, IRF RUBIRABULLEN e ALA CAPELOZZA were responsible for the literature review, design, organization and writing of the article.
REFERENCES
1. Bajuscak RE, Hall EH, Giambarresi LI, Weaver T. Bacterial contamination of dental radiographic film. Oral Surg Oral Med Oral Pathol.1993;76(5):661-3. [ Links ]
2. Bartoloni JA, Chariton DG, Flint DJ. Infection control practices in dental radiology. Gen Dent. 2003;51(3):264-71.
3. American Dental Association Council on Scientific Affairs. The use of dental radiographs: update and recommendations. J Am Dent Assoc. 2006;137(9):1304-12.
4. Silva MAS, Martins MV, Medici Filho E, Moraes LC, Castilho JCM, Jorge AOC. Evaluation of the efficiency of an infection control protocol in dental radiology by means of microbiological analysis. Cienc Odontol Bras.2004;7(3):15-21.
5. Palenik CJ. Infection control practices for dental radiography. Dent Today.2004;23(6):52-5.
6. Thomas LP, Abramovitch K. Infection control for dental radiographic procedures. Tex Dent J. 2005;122(2):184-8.
7. Pontual MLA, Ortega AI, Napimoga MH, Haiter Neto F, Gonçalves RB. Eficácia de soluções desinfetantes em filmes radiográficos periapicais. Rev Assoc Paul Cir Dent. 2004;58(1):47-51.
8. Stanczyk DA, Paunovich ED, Broome JC, Fatone MA. Microbiologic contamination during dental radiographic film processing. Oral Surg Oral Med Oral Pathol.1993;76(1):112-9. doi: 10.1016/0030-4220(93)90305-N.
9. Hokett SD, Honey JR, Ruiz F, Baisden MK, Hoen MM. Assessing the effectiveness of direct digital radiography barrier sheaths and finger cots. J Am Dent Assoc. 2000;131(4):463-7.
10. Wenzel A, Møystad A. Work flow with digital intraoral radiography: a systematic review. Acta Odontol Scand. 2010;68(2):106-14. doi: 10.3109/00016350903514426.
11. Negron W, Mauriello SM, Peterson CA, Arnold R. Crosscontamination of the PSP sensor in a preclinical setting. J Dent Hyg. 2005;79(3):8.
12. Kalathingal SM, Moore S, Kwon S, Shuster GS, Shrout MK, Plummer K. An evaluation of microbiologic contamination on phosphor plates in a dental school. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(2):279-82. doi: 10.1016/j. tripleo.2008.05.025.
13. Govindasamy V, Ronald VS, Totey S, Din SB, Mustafa WM,
13. Kalathingal S, Youngpeter A, Minton J, Shrout M, Dickinson D, Plummer K, et al. An evaluation of microbiologic contamination on a phosphor plate system: is weekly gas sterilization enough? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(3):547-62. doi: 10.1016/j.tripleo.2009.09.035.
14. White SC, Glaze S. Interpatient microbiological crosscontamination after dental radiographic examination. J Am Dent Assoc.1978;96(5):801-4.
15. Rahmatulla M, Almas K, al-Bagieh N. Cross infection in the high-touch areas of dental radiology clinics. Indian J Dent Res.1996;7(3):97-102.
16. Katz JO, Geist JR, Molinari JA, Cottone JA. Potential for bacterial and mycotic growth in developer and fixer solutions. Dentomaxillofac Radiol.1988;Suppl 10:52.
17. Bachman CE, White JM, Goodis HE, Rosenquist JW. Bacterial adherence and contamination during radiographic processing. Oral Surg Oral Med Oral Pathol. 1990;70(5):669-73. doi: 10.1016/0030-4220(90)90420-W.
18. Katz JO, Cottone JA, Hardman PK, Taylor TS. Infection control in dental school radiology. J Dent Educ.1989;53(4):222-5.
19. Ash JL, LaTurno AS, Corcoran JF. The use of a sealed plastic bag for radiographic film to avoid cross-contamination. J Endod.1984;10(10):512-4.
20. Ciola B. A readily adaptable, cost-effective method of infection control for dental radiography. J Am Dent Assoc. 1988;117(2):349.
21. Neaverth EJ, Pantera EA. Chairside disinfection of radiographs. Oral Surg Oral Med Oral Pathol.1991;71(1):116-9.
22. Packota GV, Komiyama K. Surface disinfection of saliva contaminated dental X-ray film packets. J Can Dent Assoc.1992;58(9):747-51.
23. Wenzel A, Frandsen E, Hintze H. Patient discomfort and crossinfection control in bitewing examination with a storage phosphor plate and a CCD-based sensor. J Dent. 1999;27(3):243- 6. doi: 10.1016/S0300-5712(98)00063-3.
 Endereço para correspondência:
Endereço para correspondência:
LMPSR FERNANDES
e-mail: lucianamfernandes@usp.br
Received on: 19/11/2009
Final version resubmitted on: 23/4/2010
Approved on: 24/5/2010













