Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.63 no.1 Porto Alegre Jan./Mar. 2015
CLÍNICO / CLINICAL
Mucoepidermoid carcinoma of the retromolar region: report of a clinical case
Carcinoma mucoepidermoide em região retromolar: descrição de um caso clínico
Daliana Queiroga de Castro GOMESI; Miguel Franklin Alves SILVAI; Jozinete Vieira PEREIRAI; Patrícia Meira BENTOI; Robéria Lúcia de Queiroz FIGUEIREDOI; Márcia Cristina da Costa MIGUELII
I Universidade Estadual da Paraíba, Departamento de Odontologia. Campus Universitário Bodocongó, 58109-790, João Pessoa, PB, Brasil
II Universidade Federal do Rio Grande do Norte, Departamento de Odontologia
ABSTRACT
Although the number of reported cases is low, mucoepidermoid carcinoma is the most common malignant salivary gland neoplasm in the oral cavity. Its etiology is unknown. Clinically, it is described as a painful or painless swelling most often seen in the palate. Due to its great biological diversity, treatment and prognosis depend on the histological grade, location, and tumor stage. The objective of the present study was to describe a clinical case of mucoepidermoid carcinoma in a Brown female patient aged 45 years. Intraoral physical examination revealed a 1.0 cm diameter, bluish bubble with clear boundaries in the left retromolar region. The bubble had been there for roughly four months. An excisional biopsy was performed to confirm the clinical diagnosis of mucocele. Yellowish mucous leaked during the excision. The anatomicalpathological result was mucoepidermoid carcinoma, after which the patient was referred to a head and neck surgeon. Hence, we emphasize the importance of early diagnosis and proper management of this disease. Even when its clinical appearance is not suggestive of malignancy, mucoepidermoid carcinoma diagnosis should be considered in cases of proliferative oral lesions.
Indexing terms: Diagnosis. Minor salivary glands. Mucoepidermoid carcinoma.
RESUMO
Embora apresente uma casuística baixa, o Carcinoma Mucoepidermoide é a neoplasia maligna de glândula salivar mais observada na cavidade oral. Possui etiopatogenia desconhecida e, clinicamente, apresenta-se como tumefação sintomática ou não, sendo o palato, o sítio de predileção. Em decorrência da sua grande diversidade biológica, o tratamento e prognóstico dependem do grau histológico, da localização e do estágio clínico do tumor. O presente trabalho teve por objetivo descrever um caso clínico de carcinoma mucoepidermoide de uma paciente do gênero feminino, 45 anos de idade, feoderma. O exame físico intraoral evidenciou, em região retromolar esquerda, bolha de limites nítidos, medindo cerca de 1,0 cm de diâmetro, coloração azulada, presente há aproximadamente quatro meses. Diante do diagnóstico clínico de mucocele, foi realizada a biopsia excisional; durante a execução da mesma, observou-se extravasamento de muco amarelado. Após o resultado anatomopatológico de carcinoma mucoepiderrmoide, a paciente foi encaminhada ao cirurgião de cabeça e pescoço. Desta forma, enfatiza-se a importância do diagnóstico precoce e correto manejo desta patologia, que, mesmo quando sua aparência clínica não sugerir malignidade, deve ser considerada como hipótese diagnóstica em lesões proliferativas da boca.
Termos de indexação: Diagnóstico. Glândulas salivares menores. Carcinoma mucoepidermoide.
INTRODUCTION
Malignant neoplasms of the salivary glands are rare and represent 3 to 5% of all malignant tumors that occur in the head and neck region. They may affect the major or minor salivary glands1-2.
Mucoepidermoid carcinoma (MEC), the most frequent malignant salivary gland neoplasm in the oral cavity, was studied and described as a distinct entity for the first time by Stewart et al.3. Although its etiology is unknown, in some cases it may be associated with genetic factors or exposure to radiation and/or smoking2. This epithelial tumor derives from the reserve cells of the excretory duct, whose biological behavior varies from low to high grade4. Although all grades are capable of metastasis, the low-grade tumors are generally locally invasive but lowly aggressive. The neoplasm can infiltrate in the neighboring tissues or develop distant metastases in the lungs, bones, and brain5. The tenyear survival rates for lowly and highly malignant tumors are 90% and 42%, respectively6.
The mean age of affected individuals is 45 years. The most common intraoral locations of the tumor are the palate, buccal mucosa, and alveolar region7. Regional lymphadenopathy is uncommon. Clinically, the lesions are nodular, consistent, and stationary, evolve slowly, grow usually asymptomatically, have variable sizes, and may ulcerate. The color of the lesion varies from blue to red or purple8.
Histologically, MEC consists of a mixture of mucous, squamous (epidermoid), and intermediate cells whose malignancy is determined by their histological graduation, including cystic and solid lobular arrangements permeated by a fibrous connective tissue stroma5.
Based on its histological characteristics, infiltration capacity, local recurrence, and morbidity, MEC is classified into three grades of malignancy: low grade when cell atypia is minimal, cystic formation is prominent, and proportion of mucous cells is high; high grade when there is considerable pleomorphism and mucous activity and high proportion of quickly growing squamous cells, causing early pain, possible ulceration, bone resorption, lymphadenopathy, and even facial paralysis; and intermediate grade when the three types of cells are present but intermediate cells prevail2,9-10.
The harmless appearance of the neoplasm hinders the differential diagnosis of other benign lesions, such as salivary retention cysts, hemangioma, nevus, or other cystic processes11.
The location, histological grade, and tumor stage determine the treatment plan and prognosis. The diagnosis is based on an association between the clinical findings and the complementary tests that include imaging, fine-needle aspiration biopsy (FNAB), and anatomical-pathological examination of the biopsy specimen9,12-13.
Lesions of the oral mucosa, floor of the mouth, lips, and retromolar region resemble asymptomatic submucous masses, while lesions of the tongue are usually painful14.
Generally, treatment consists of total excision of the lesion, including partial or complete removal of the compromised gland, and depending on the extension of the lesion and histopathological grade, possibly postoperative radiotherapy3. Radical neck dissection is also performed in patients with clinical evidence of metastasis and in highly malignant cases10.
The present study aimed to describe a clinical case of MEC in a Brown female patient aged 45 years seen at the Hospital Napoleão Laureano, João Pessoa (PB).
CASE REPORT
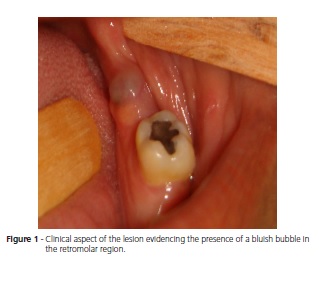
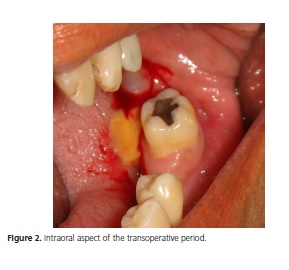
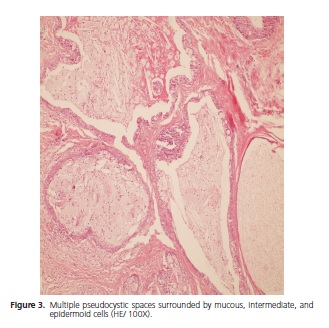
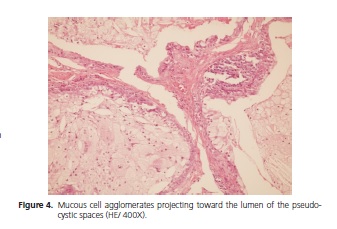
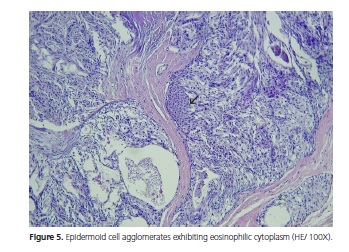
The patient is a Brown female aged 45 years from the city of João Pessoa (PB). She visited the Stomatology Outpatient Clinic of Hospital Dr. Napoleão Laureano complaining of "uncontrollable gingival bleeding" that had been going on for one year and eight months. The patient denied having systemic problems but had undergone mastectomy, radiotherapy, and chemotherapy to treat a malignant breast neoplasm seven years earlier. Extraoral physical examination revealed no change or lymphadenopathy. Intraoral physical examination evidenced hyperemic gum that bled when touched and a biofilm on the dental surfaces, the main complaint of the patient. The left retromolar region contained an exophytic, flaccid, bluish, sessile, bubbly lesion with smooth surface, clear borders, and an approximate diameter of 1.0 cm (Figure 1). The painless lesion had been present for four months and did not bleed. A radiograph revealed no bone changes. The lesion was diagnosed as mucocele and excisionally biopsied. A yellowish mucous leaked during the excision (Figure 2). The specimen was sent to anatomicalpathological analysis. The histological cuts were stained with hematoxylin and eosin, revealing a fragment of a malignant salivary gland neoplasm with proliferation of three cell types: polygonal epidermoid cells, eosinophilic cytoplasm, and vesicular nucleus; mucosa exhibiting ample clear cytoplasm and basaloid intermediate cells, scarce eosinophilic cytoplasm, and more hyperchromatic nucleus. These cells surrounded pseudocystic spaces of different sizes and formed agglomerates that projected to the inside of the pseudocystic lumens (Figure 3 and 4). Alternatively, eosinophilic substances and cells with granular cytoplasm filled the pseudocystic spaces. The epidermoid cells were more cohered and sometimes presented numerous and prominent nucleoli. Lightcolored cells were present among this neoplastic proliferation. The stroma contained vascularized fibrous connective tissue of variable density, with foci of mostly mononuclear moderate inflammatory infiltrate. Fragments of salivary gland, muscle, and fat tissues, nerve fascicles, and hemorrhagic extravasation completed the histological picture. Thus, the histopathological diagnosis was MEC with unspecified histological graduation since the margins were compromised. The patient was referred to a head and neck surgeon who opted for observation despite the histologically compromised margins because of the lack of neoplastic evidence. The patient was followed for one year and five months without clinical evidence of tumor recurrence. However, since the patient complained of burning in the region, especially when eating, we opted for surgery. The histopathological result confirmed the excisional biopsy diagnosis, MEC (Figure 5). Radiotherapy was not performed because the tumor was a low-grade MEC. The patient has been followed by the head and neck surgeon for about four months without complaints or clinical evidence of the lesion. The patient signed an informed consent form granting full use of the data reported herein.
DISCUSSION
The embryological, histological, and anatomic characteristics of the oral cavity associated with environmental factors provide numerous opportunities for the development of asymptomatic or symptomatic lesions.
The present article reports a case of MEC in a female patient. Until now, there is no consensus regarding the preferred gender of this neoplasm. Some believe there is no preference14, while others believe MEC is more prevalent in women (60.2%)5,1,15.
The clinical diagnosis of mucocele was based on the finding of a bluish, flaccid, bubbly lesion with smooth surface and clear borders accompanied by mucous content seen during the excisional biopsy. MEC is usually bluish and unattached, like mucocele3. The presence of mucous secretion in lesions with these characteristics would sustain the diagnostic hypothesis of mucocele5. Some complementary tests are necessary for proper diagnosis, usually an incisional biopsy3,16-18. MEC's benign clinical appearance usually leads to a diagnosis of pleomorphic adenoma or other lesions, including mucous retention cyst, hemangioma, pigmented nevus, and cystic processes19.
In this case, the patient had no pain, lymphadenopathy, or bone changes in the radiograph, further hindering the clinical diagnosis. The absence of symptomatology can delay diagnosis, making treatment less effective5. Additionally, pain is not always present in MEC cases20. However, there are reports of symptomatic cases associated with lymphadenopathy, ulceration, or bone involvement21.
The present MEC was located in an infrequent location. The major salivary glands are affected more often, usually represented by the parotid22-23. Kolude24 analyzed 34 MEC patients and found that only 25% of the lesions affected the minor salivary glands, most of which in the palate. The same was observed by Dedivitis25. Other oral areas affected in decreasing order are the buccal mucosa, alveolar mucosa, tongue, retromolar region, floor of the mouth, and lips5.
Moreira26 observed that the parotid gland is the most frequent site of malignant lesions, followed by the submandibular and minor salivary glands distributed throughout the oral cavity. However, Ledesma-Montes & Graces-Ortiz27 reported that the palate was affected most often.
Isolated tumors in the retromolar trigone are rare because of its small space. In general they are squamous cell carcinomas with rare exceptions. The lesions frequently extend to the tonsils, anterior pillar, and soft palate28.
In the present study the excisional biopsy specimen was sent to anatomical-pathological analysis. This procedure favors early diagnosis, improves the prognosis, and increases the odds of a successful treatment. MEC, as well as any other lesion of the maxillomandibular complex, should preferably be diagnosed as early as possible, usually improving the prognosis3. Therefore, the diagnosis of a malignant lesion in the initial phase has a favorable impact on its treatment29.
The stage of the lesion upon diagnosis is important because more advanced cases require more complex treatments, and the prognosis is poor30. In most oral tumor cases, the diagnosis is late since patients look for experts only when the lesions are very advanced or inoperable. This is unacceptable since the oral cavity is easy to access and inspect, so late diagnoses are not justified6.
Dentist sensitization and training regarding the thorough examination of the stomatognathic system is critical to promote early diagnosis and prevent oral lesions, consequently requiring less invasive surgeries that result in better quality of life for the patient6.
CONCLUSION
Given the above, we emphasize the importance of dentists knowing the symptomatology of mucoepidermoid carcinoma for early diagnosis and appropriate treatment. MEC should be considered a diagnostic hypothesis in proliferative oral lesions, even when its clinical appearance does not suggest malignancy.
Collaborators
DQC GOMES and MFA SILVA conceived and designed the study and wrote the article. JV PEREIRA, PM BENTO, RLQ FIGUEIREDO, and MCC MIGUEL analyzed and interpreted the data and wrote the article.
REFERENCES
1. Ito FA, Ito K, Vargas PA, de Almeida OP, Lopes MA. Salivary gland tumors in a Brazilian population: a retrospective study of 496 cases. J Oral Maxillofac Surg. 2005;34:533-6. doi:10.1016/j. ijom.2005.02.005 [ Links ]
2. Antunes AA, Antunes AP. Tumores das glândulas salivares maiores: estudo retrospectivo. Rev Bras Patol Oral. 2005;4(1):2-7.
3. Zini M, Moreschi E, Trento CL, Gottardo VD, Zardetto RJr, Aleixo TRC. Carcinoma mucoepidermoide em palato: relato de caso. Rev Cir Traumatol Buco-Maxilo-Fac. 2010;10(1):57-62.
4. Akrish S, Peled M, Ben-Izhak O, Nagler R. Malignant salivary gland tumors and cyclo-oxygenase-2: a histopathological and immunohistochemical analysis with implications on histogenesis. Oral Oncology. 2009;12(45):1044-50. doi: 10.1016/j.oraloncology.2009.07.016
5. Giovanini EG, Simonato LE, Castro EVFL, Soubhia AMP, Castro AL. Carcinoma mucoepidermóide de palato: descrição de um caso clínico. RFO. 2007;12(1):61-4.
6. França DCC, Novelli EVA, Ferrarini TFN, Monteiro AD, Silva AAS, Aguiar SMHCA. Carcinoma mucoepidermóide: relato de caso. Rev Odontol Araçatuba. 2008;29(2):20-3.
7. Gassler N, Erbe M, Caselitz J, Donner A. Mucoepidermoid carcinoma of palatinal glands with exuberant foreign-body giant cell reaction. Pathol Res Pract. 2008;204(9):689-91. doi: 10.1016/j.prp.2008.04.003
8. Choi D, Kim H, Lee KS, Lee KG, Park CK. Mucoepidermoid carcinoma of the liver diagnosed as a liver abscess: report a case. Surg Today. 2004;34(11):968-72. doi: 10.1007/s00595-004-2820-7
9. Sobral APV, Kowalski LP, Araújo NS, Araújo VC. Gradação histológica do carcinoma mucoepidermóide em glândulas salivares maiores e menores. Rev Pós-Grad. 2001;8(4):334-8.
10. Neville BW, Damm DD, Allen CM, Bouquot JE. Patologia oral e maxilofacial. Rio de Janeiro: Guanabara Koogan; 2009.
11. Puricelli E, Ponzoni D, Peschke R, Baraldi CE. Carcinoma mucoepidermóide no palato: revisão da Literatura e relato de um caso em paciente jovem. Rev Fac Odontol. 1998;39(2):8-10.
12. Santos GC, Martins MR, Pellacani LB, Vieira ACT, Nascimento LA, Abrahão M. Neoplasias de glândulas salivares: estudo de 119 casos. J Bras Patol Med Lab. 2003;39(4):371-5. doi: 10.1590/ S1676-24442003000400016
13. Tinoco P, Pereira JCO, Lourenço RCF, Brito TSC, Pereira BM, Carrara VL, et al. Carcinoma mucoepidermoide de glândulas salivares menores. Arq Int Otorrinolaringol. 2011;15(1):99-101. doi: 10.1590/S1809-48722011000100016
14. Pires FR, Alves FA, Almeida OP, Kowalski LP. Carcinoma mucoepidermóide de cabeça e pescoço: estudo clínico-patológico de 173 casos. Rev Bras Otorrinolaringol. 2002;68(5):679-84.
15. Salazar CM, Saa J, Sánchez-Jara MR, García JL, González M. Carcinoma mucoepidermóide de vestíbulo nasal. Acta Otorrinolaring Esp. 2000;51(7):729-32.
16. Mello-Filho FV, Brigato RR, Mamede RCM, Ricz HMA, Saggioro FP, Xavier SP. Central mucoepidermoid carcinoma: Report of 2 cases. Br J Oral Maxillofac. 2008;46(3):239-41. doi: 10.1186/1477-7819-1-1
17. Perez DEC, Pires FR, Alves FA, Lopes MA, Almeida OP, Kowalski LP. Juvenile intraoral mucoepidermoid carcinoma. J Oral Maxilofac Surg. 2008;66(2):308-11. doi: 10.1016/j.joms.2007.04.029
18. Woo HJ, Bai CH, Kim YD, Song SY. Mucoepidermoid carcinoma of the submandibular gland after chemotherapy in a child. Auris Nasus Larynx. 2009;36(2):244-6. doi:10.1016/j.anl.2008.05.003
19. Puricelli E, Ponzoni D, Peschke R, Baraldi CE. Carcinoma mucoepidermoide no palato: revisão de literatura e relato de um caso em paciente jovem. Rev Fac Odontol. 1998;39(2):8-10.
20. Hyan DM, Veness MJ, Morgan GJ. Minor salivary gland carcinoma involving the oral cavity or oropharynx. Aust Dental J. 2004;49(1):16-9. doi: 10.1111/j.1834-7819.2004.tb00044.x
21. Epstein JB, Hollender L, Pruzan SR. Mucoepidermoid carcinoma in a young adult: recognition, diagnosis, and treatment and responsibility. Gen Dent. 2004;52(5):434-9. doi:10.1016/j. rpemd.2011.11.002
22. Ribeiro KC, Kowalski LP, Saba LM, Camargo B. Epithelial salivary glands neoplasms in children and adolescents: a forty-fouryear experience. Med Pediatr Oncol. 2002;39:594-600. doi: 10.1002/mpo.10168
23. Antunes AA, Antunes AP. Tumores das glândulas salivares maiores: estudo retrospectivo. Rev Bras Patol Oral. 2005;4(1):2-7.
24. Kolude B, Lawoyin JO, Akang EE. Mucoepidermoid carcinoma of the oral cavity. J Nat Med Assoc. 2001;93:178-84.
25. Dedivitis RA, Júnior Pfuetzenreiter EG. Abordagem dos tumores de glândulas salivares menores. Rev Bras Cir Cabeça Pescoço. 2006;35(4):214-6.
26. Moreira ARO, Oliveira CDM, Figueirêdo EP, Silva RR, Lopes FF, Bastos EG. Levantamento epidemiológico das enfermidades das glândulas salivares em São Luís - MA: casuística de vinte anos. RFO. 2009;14(2):105-10.
27. Ledesma-Montes C, Garces-Ortiz M. Salivary gland tumours in a Mexican sample: a retrospective study. Med Oral. 2002;7:324-30.
28. Konno S N. Neoplasias da cavidade oral e da orofaringe [citado 2011 Nov 10]. Disponível em: <http://www.forl.org.br/pdf/ seminarios/seminario_57.pdf>.
29. Alves ATNN, Maia ABP, Libório AO, Piasesi JL, Souza CB, Ribeiro BF. Diagnóstico precoce e prevenção do câncer oral: um dever do cirurgião-dentista. Rev Bras Odontol. 2002;59(4):259-60.
30. Martins MAT, Marques FGOA, Pavesi VCS, Ramão MMA, Lascala CA, Martins MD. Avaliação do conhecimento sobre o câncer bucal entre universitários. Rev Bras Cir Cabeça Pescoço. 2008;37(4):191-7.
 Correspondence to:
Correspondence to:
MCC MIGUEL
e-mail: mccmiguel@hotmail.com
Received on: 10/5/2012
Final version resubmitted on: 30/7/2012
Approved on: 30/8/2012













