Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.63 no.4 Porto Alegre Out./Dez. 2015
ORIGINAL / ORIGINAL
Photoinactivation of Candida albicans using methylene blue as photosensitizer
Fotoinativação de Candida albicans utilizando azul de metileno como fotossensibilizadorI
João Nilton Lopes de SOUSAII; Bruna Honório de QUEIROGAIII; Patrícia de Oliveira KOCERGINSKYIV; Petrusk Homero Campos MARINHOIV; Ângela Toshie ARAKIV
I Artigo baseado na tese intitulada "Inativação de células planctônicas de Candida albicans empregando terapia fotodinâmica". Universidade Cruzeiro do Sul; 2014
II CUniversidade Federal de Campina Grande, Faculdade de Odontologia, Departamento de Periodontia
III Universidade Federal de Campina Grande, Faculdade de Odontologia. Patos, PB, Brasil
IV Faculdades Integradas de Patos, Departamento de Biomedicina. Patos, PB, Brasil
V Universidade Cruzeiro do Sul. São Paulo, SP, Brasil
ABSTRACT
Objective
To evaluate the effect of photodynamic therapy in the inactivation of Candida albicans planktonic cells, using methylene blue, 150 mg/mL, as a photosensitizer.
Methods
Aliquots of 100 (μg/mL of the fungal suspension in a 106 cells/mL concentration were seeded in microtiter plates with 96 wells, where the same volume of methylene blue was deposited, remaining for 5 min pre-irradiation. Then, the low power laser light was applied (wavelength: 660 nm, power: 100 mW and dose: 426 J/cm2) for 128s using a portable semiconductor laser. Control experiments were performed without illumination and in the absence of methylene blue, and another in the presence of methylene blue without illumination and with illumination, replacing the photosensitizer by saline. In each experimental condition, serial dilutions (10-1 to 10-3) were obtained and 25 μL aliquots were seeded in Sabouraud Dextrose Agar duplicate. After this period, the number of colony forming units per milliliter (CFU/mL) was determined and the data were submitted to variance analysis and Kruskal Wallis test (p <0.05).
Results
At the concentration of 150 μg/mL, and time of 5 min incubation, the reduction was significant (p˂0.05). In the absence of irradiation, methylene blue produced no reduction in CFU/mL.
Conclusion
Photodynamic Therapy presented antifungal effect against Candida albicans and can be used as an adjunct to conventional treatment.
Indexing terms: Candida albicans. Methylene blue. Photochemotherapy.
RESUMO
Objetivo
Avaliar o efeito da terapia fotodinâmica na inativação de células planctônicas de Candida albicans, empregando azul de metileno a 150 μg/ mL como fotossensibilizador.
Métodos
Alíquotas de 100 μg/mL da suspensão do fungo na concentração de 106 células/mL foram semeadas em placas de microtitulação com 96 poços, onde foi depositado o mesmo volume de azul de metileno, permanecendo por um período de pré-irradiação de 5 min. Em seguida, aplicou-se luz laser de baixa potência (comprimento de onda: 660 nm, potência: 100 mW e dose: 426 J/cm2) por 128s, utilizando um Laser semicondutor portátil. Experimentos controles foram realizados, sem iluminação e na ausência de azul de metileno, outro na presença de azul de metileno sem iluminação e com iluminação, substituindo o fotossensibilizador por solução salina. De cada condição experimental, diluições em série (10-1 a 10-3) foram obtidas e alíquotas de 25 μL foram plaqueadas em duplicata em Agar Sabouraud Dextrose. Após este período, o número de unidades formadoras de colônias por mililitro (CFU/mL) foi determinado e os dados foram submetidos à análise de variância e teste de Kruskal Wallis (p <0,05).
Resultados
Na concentração de 150 μg/mL e tempo de 5 min de incubação, a redução foi significativa (p<0,05). Na ausência de irradiação, o azul de metileno não produziu redução de CFU/mL.
Conclusão
A Terapia Fotodinâmica apresentou efeito antifúngico contra Candida albicans, podendo ser utilizada como coadjuvante ao tratamento convencional.
Termos de indexação: Candida albicans. Azul de metileno. Fotoquimioterapia.
INTRODUCTION
Opportunistic fungi can cause critical superficial or invasive infections in apparently healthy patients and especially in debilitated or immunocompromised patients. Due to difficulties in diagnosis and the limited availability of effective antifungal drugs, diseases caused by fungi represent a growing threat to human health, and can result in highly attributable mortality1.
Candida albicans is an opportunistic fungus, which requires some predisposing factors for the disease development, such as immunosuppressive agents, use of broad-spectrum antibiotics, xerostomia, prosthetic use and poor oral hygiene. In patients with Acquired Immunodeficiency Syndrome (AIDS), the oral candidosis is the most common fungal manifestation, as an indicator of the infection progression2.
According to Lam et al.3, the infections caused by fungi represent a critical challenge to public health with high socio-economic importance, mainly due to traditional antifungal resistant species, requiring the development of scientific research that seek to expand the lesions treatment forms, enabling the effective alternative treatment of failure cases and improving the well-being of patients affected by this disease.
Photodynamic Therapy (PDT) is a treatment already used in several medical specialties, such as dermatology, oncology and ophthalmology, and has been investigated as an antimicrobial therapy, which consists in the administration of a photosensitizing (FS) dye, followed by the lesion illumination with visible light, forming cytotoxic species in the presence of oxygen, such as the singlet oxygen and free radicals4. These reactive chemical species are mostly toxic and can cause: destruction of proteins, lipids, nucleic acids, and other cell components, resulting in the destruction of microbial cells5.
Photodynamic therapy offers antimicrobial action mechanisms different from antifungal drugs, which attenuates the resistance development cases, making this treatment modality an effective alternative against C. albicans strains resistant to conventional therapy6. The use of methylene blue in photodynamic therapy has shown effective results in Candida albicans inactivation, however, it is necessary to establish a suitable clinical protocol regarding the pre-irradiation time and the dye concentration, without toxic effects to the organism cells.
Therefore, this study aims to evaluate, in vitro, the photosensitizing effects in Candida albicans with laser radiation, using methylene blue as a photosensitizer.
METHODS
Candida albicans (ATCC10231) strains were used, acquired from the tropical crops collection (CCT) of André Tosello Foundation. For the performing the experiments, the Agar Sabouraud Dextrose-ASD (União Química, São Paulo, Brazil) culture medium was used with 5 μg/mL chloramphenicol, as preconized in Pereira et al.7.
Photosensitizer (FS) preparation and incubation time
The FS agent used was methylene blue (AM) PA (Sigma-Aldrich, São Paulo, Brazil). Stock solutions from AM PA, at a concentration of 300 μg/mL were prepared by dissolving the powder in sterile saline solution (0.85% Na Cl) at pH 78. During the experiment, in microtiter plate wells, 100 μL of FS stock solution, at room temperature, was again diluted by adding 100 μL of Candida albicans suspension in saline solution 0.85%9, resulting in a final volume of 200 μL. As the well volume is duplicated (100 μL of FS and 100 μL of cell suspension), FS concentration is halved: 150 μg/mL (work concentration). Then, the AM dye was in contact with the Candida albicans cells, in the dark and at room temperature, before the illumination, for an incubation period of 5 minutes (pre-irradiation time). With this purpose, the plates were in an environment without luminosity and involved by an aluminum foil.
Irradiation source for photodynamic therapy (PDT)
The light source was a portable Semiconductor Laser (Laser DUO®, GaAlAs, InGaAlP, λ880nm and λ660nm, MM OPTICS Ltda., São Carlos, Brazil). This instrument features constant output power of 100 mW and the laser beam area of 3 mm2, which matches with the opening area of each of the 96 U-deep wells of the microtiter plates used in this study. For the irradiation protocol, the equipment was adjusted to a wavelength of 660 nm, which corresponds to the AM absorption range, and to the illumination time of 128s, resulting in an energy influence of 426 J/cm2. Prior to the experiments, the laser was calibrated using a device (Check MM OPTICS Ltda., São Carlos, Brazil). Before each irradiation, the instrument tip was immersed in chlorhexidine digluconate solution to 2% for 5 minutes to promote its disinfection. After this period, the antimicrobial agent was removed from the device by means of irrigation with 10 mL of saline solution (0.9%) and dried with sterile gauze. The equipment tip was positioned perpendicular to the wells opening and the irradiation was carried out with 90° incidence angle. In order to avoid a possible cumulative effect due to irradiation spreading, each sample was individually irradiated and collected9.
Inoculum preparation of Candida albicans
Candida albicans (ATCC 10-231) cells were sub cultivated from stocks flasks in Agar Sabouraud Dextrose- ASD under aerobic conditions at 37ºC. After 24 h incubation, a sample of colonies was removed from the Agar plate surface and suspended in sterile saline solution 0.85% of NaCl10. After stirring using a Vortex device (Vision®) for 2 minutes, the cell suspension turbidity was adjusted using a spectrophotometer to 530 nm to obtain suspensions with optical density of 0.284, which corresponds to fungi concentration of 106 colony forming units per mL (CFU/mL)4,7,9.
Experimental conditions
All tested conditions were carried out in the dark at room temperature and under sterile conditions in a laminar flow chamber. Microtiter plates were covered with aluminum foils to prevent the environment light penetration.
Photodynamic Therapy Group (PDT Group)
In photodynamic therapy group, PDT group (FS+ L+), Candida albicans strains were sensitized with the FS, methylene blue, and exposed to the laser (L). With constant stirring, 100 μL aliquots of fungus suspension (106 CFU/ mL) were individually transferred to a microtiter plate well with 96 wells. Then, the same volume of AM (100μl) was added at the tested concentration (300 μg/mL), resulting in a final volume of 200 μL per well and final concentration of 150 μg/mL (PDT AM group - 150 μg/ml). Subsequently, the plates containing the resulting suspensions were left at rest, in the dark, during the incubation time of 5 minutes. After the pre-irradiation time, the plates were opened and yeast cells were continuously irradiated, from the top of the microtiter plate, with a diode laser (wavelength of 660nm; 100 mW power, and dose of 426 J/cm2) for 128s. The wells content was duly homogenized before illumination and sampling.
Methylene Blue Group (AM Group)
In AM group, FS effect alone, on Candida albicans cells inactivation, was tested by applying the solution following the same protocol established for the PDT Group (FS+ L+), especially regarding the volume (100 μL), tested concentration (150 μg/mL), and incubation time (5 min.) with the absence of light, but without the laser treatment. In this group, it was possible to evaluate the AM toxicity in Candida albicans cells.
Laser Group (L Group)
In the laser group, L group, Candida albicans strains were exposed to laser light (L), but have not been sensitized by FS. With this group it was possible to evaluate the laser light effect in isolation on yeast cells inactivation. 100 μL aliquots of cell suspension were individually transferred to one of the wells of a microtiter plate. Then, the same volume of sterile saline solution 0.85% was transferred to the well. The test system was kept in the dark by the same incubation time of the previous groups (5 min.).
Saline solution group (SS Group)
In the saline solution group, SS group, Candida albicans cells did not receive any treatment, i.e. were sensitized by FS, or exposed to light. 100 μL aliquots of the suspension (106 CFU/mL) were transferred to the wells of a microtiter plate, and then, the same volume of saline solution 0.85% was deposited in the well and the plate was at rest in the dark by the same incubation time of previous groups (5 minutes). The results obtained with this sample cultures were used as parameter for comparison with those obtained with cultures of samples submitted to the experimental conditions.
Viability evaluation of Candida albicans cells
For all conditions evaluated, 1:3 serial dilutions were made from the samples contained in the plates' wells. For this, a 100 μL aliquot was removed from each well and transferred to a test tube containing 900 μL of sterile saline solution (0.85%). This tube was vigorously stirred in a tube stirrer (Vortex-Vision®) and a new aliquot of 100 μL was removed from it and put into another test tube containing 900 μL of sterile saline solution. This procedure was performed three times for each sample and, therefore, serial dilutions from 10-1 to 10-3 were obtained. Serial dilutions (10-1, 10-2 and 10-3) are used for the seeding in Petri dishes containing Agar Sabouraud Dextrose culture medium.
Then, 25 μL aliquots of each serial dilution were pipetted in duplicate. Additionally, 25 μL aliquots were removed from the plates well and directly transferred the Petri dishes, without dilution. A sterile Drigalsky handle was used to spread the solution over the culture medium on the plate. Also, the seeding procedures were performed in duplicate. After 48 hours of incubation at 37ºC, the Petri dishes related to the evaluated experimental conditions samples were submitted to the colonies count. For this procedure, the colonies quantification was performed and the numbers of colony-forming units of were calculated.
CFU/mL Count Technique
The plates reading was made by default count of Candida albicans colonies (Colony Forming Unit - CFU/mL) using a colony counter. For the counting, plates containing a number of colonies that was within the accuracy and repeatability range from 30 to 300 colonies were selected. For each dilution (10-1; 10-2 and 10-3), the colonies number results was obtained by the arithmetic mean of the duplicate plates results and multiplied by the corresponding dilution (10, 100 or 1000). The result of each group was found by using the arithmetic mean of the three dilutions. Then, to find the CFU number per mL, the result of each group was converted from the inoculated amount of 25 μL to 1 mL, dividing the result by 0.025, getting the final result of CFUs/mL in the potency of 10. For the CFU/mL count analysis of Candida albicans, it was decided to transform values into potency of 10, using the decimal logarithm of this number for the statistical analysis.
In situations where all the plates showed less than 30 colonies, the result was expressed by the number of colonies of the lesser dilution plate. When all the plates showed more than 300 colonies in the greater dilution and it was possible to perform the count, the result was found by multiplying the value in the colonies number in each plate by the respective dilutions, and then, calculating the arithmetic mean. When the number of colonies was excessively high to count, a representative portion of the colonies distribution across the plate was chosen and the number of colonies present was estimated.
Statistical analysis
The log10 (CFU/mL) results for each tested condition was compared using Kruskal-Wallis statistical test and in the case of a significant difference, multiple comparisons of such test were used. P values less than 0.05 were considered significant. The choice of Kruskal-Wallis test was due to the number of groups. The data were entered in the EXCEL spreadsheet and the software used to obtain the statistical calculations was the Trial version in Portuguese. The results of this research were expressed through the statistical measures: mean, standard deviation (SD), and median.
RESULTS
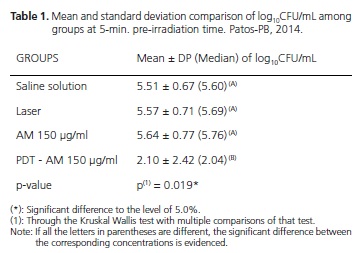
According to table 1, it can be noticed that in 5-minutes incubation, the group irradiated with laser light, associated with the concentration of 150 μg/mL of methylene blue as photosensitizer (PDT AM group), showed a lower mean in the log10 CFU/mL value, p = 0.019, showing inhibitory effect in Candida cells, compared to other groups, which showed no inhibitory effect of Candida Albicans cells. When comparing the CFU/mL count results among the groups that assessed the methylene blue (AM Group) toxicity in the dark and light in the dose used (Laser group), with the group that received no treatment (Saline solution group), it was observed that only the AM or only the light did not exercise inhibitory effect against Candida albicans cells.
DISCUSSION
The light therapeutic properties studies with antimicrobial purpose are primarily motivated by resistance cases of conventional antimicrobial3,6,11-14. The need to overcome these deficiencies led to the exploration of alternative treatments and unconventional approaches to the control of microbial infections14.
Currently, the light-based antimicrobial treatment is called Photodynamic therapy4-5,15. The three main components of PDT are visible light, FS and oxygen16. Due to the fact that they absorb light with high efficiency, in some region of the visible spectrum, some of the FSs are able to induce or participate in photochemical reactions and produce reactive oxygen molecules5,14,17-18.
Several studies that evaluated azole-resistant Candida species, noted that these strains were sensitive to PDT11-12,19-20. The photosensitizer and light application specifically on the site affected by candidiasis, dramatically reduces the local and systemic side effects, allowing the photodynamic therapy to be seen as a promising treatment against Candida albicans21.
The light sources used more often in PDT against Candida albicans were LED12 and the low-intensity laser, highlighting the diode laser6,8-9,10,22. In this research, diode laser of MM OPTICS (Laser DUO®) was used and, due to being portable and having batteries, this presents a greater advantage compared to the handling without relying on an energy source, and can be applied to patients in several environments: domestic, clinic, family health care units, or intensive care units.
Methylene blue was the photosensitizer used in this study because it is a low toxicity dye with high visible light absorption at wavelengths greater than 620 nm6. It is used with different purposes in microbiology and pharmacology for quite some time and, lately, has been applied on PDT as a photosensitizing drug in association with a continuous light source. So that the photosensitizer can be one light-activated by laser, the light applied must present a specific wavelength6,20. In this study, the red light with a wavelength of 660 nm was used, corresponding to the absorption spectrum of methylene blue, and was adjusted to the appliance before illumination.
The studies of Dai et al.1, Kato et al.4, Teichert et al.6, Pereira et al.7, Souza et al.8, Queiroga et al.9, Munin et al.10, Silva Martins et al.17, Giroldo et al.22 and Souza et al.23, investigated the effects of photodynamic therapy on the viability of Candida albicans using methylene blue as a photosensitizer. The results of these studies proved the antifungal effect of this treatment modality. However, some points still needed to be better clarified, before recommending it for clinical therapy. There is need to establish protocols with well-defined parameters, such as the appropriate dosimetry of FS and light, and the time in which the dye must remain in contact with the microorganism13.
The experimental model chosen was microtiter plates with 96 U-deep wells. This methodology has been widely used in researches that assessed the antimicrobial effect of PDT on microorganism in plankton form from a cell suspension9,10,23.
The photo-dynamic effect depends on the FS concentration used, combined with irradiation parameters that activate the dye4,15. The studies that evaluated PDT effect on Candida albicans used different concentrations of methylene blue, from 10 μg/mL22 to 500 μg/mL6 and the most used incubation time was 5 minutes7-9. Therefore, to respond to the PDT effect hypothesis on the Candida albicans cells, this study used the concentration of 150 μg/ mL and the incubation time of 5 minutes.
The fact that most FSs are both fluorescent and photochemically active, allows that various strategies and protocols can be combined in photodynamic therapy. The most important factor governing the PDT results is how the FS interacts with the cells in the tissue or target lesion, and the key aspect of this interaction is related to the FS intracellular location. The incubation time is required to allow the drug to be absorbed by the microorganism and accumulate in the cell structures20, such as mitochondria, lysosomes, endoplasmic reticulum, Golgi apparatus and plasma membrane3. During irradiation, it is necessary that FS is already located inside the cell, because the singlet oxygen, formed after light application, presents minimal diffusion (100nm) and cytotoxic effect only in the place where it is produced15.
As observed in Souza et al.8 and Queiroga et al.9 studies, laser light irradiation of Candida albicans, in the absence of AM, showed no influence on the microorganisms' viability. In the absence of light, methylene blue showed no cytotoxic effect on Candida albicans cells, corroborating with the aforementioned studies. Both the photosensitizer and the light are relatively harmless by itself, but when combined, in the presence of oxygen, cause selective destruction on the affected area17,22. According to Pereira & Maisch14 and Ryskova et al.15 photosensitizing agents show no or minimal inherent toxicity and the cytotoxic effect only occurs after its illumination with specific wavelength light.
In the concentration of 150 μg/mL, the CFU/ mL reduction was significant at 5-minutes incubation, compared to control groups, stating that the photodynamic therapy had effect on the cells. Therefore, according to this study parameters and the cost-benefit ratio regarding the drug concentration and the clinical procedure time, the PDT protocol proved effective when used the 5-minutes incubation and the methylene blue concentration of 150 μg/mL. Using the 5-minutes incubation and methylene blue concentrations of 100μg/mL, 150μg/mL and 50 μg/ mL, Pereira et al.7, Queiroga et al.9 and Giroldo et al.22, respectively, observed a significant reduction in cell viability of Candida albicans species.
While evaluating the studies that have applied the methylene blue as a photosensitizer, a great shortage of controlled and randomized clinical trials, using antifungal treatment against Candida albicans by photodynamic therapy was noticed, which, despite the animal and in vitro studies being very promising, it makes the clinical indication of this therapy on scientific evidence-based Dentistry impracticable. Therefore, photodynamic therapy still requires that clinical trials are designed to give it support and therapeutic applicability.
The limitation of this study was the fact that it only used a single species of Candida and only one concentration of methylene blue. However, this methodology has met the experiment initial objectives. Other studies may be developed using this method to evaluate the PDT effect against all species of Candida or a clinical trial testing different concentrations of methylene blue and different incubation times.
CONCLUSION
It was concluded that the methylene blue photosensitizer showed no cytotoxicity in the dark on Candida albicans, and also the laser light has not altered the microorganism cell viability in the photosensitizer absence. And that the photodynamic therapy decreased the CFU/mL number of Candida albicans, showing satisfactory antifungal effect against this microorganism. The 5-minutes incubation presented effective results reducing the log10 CFU/mL number.
Collaborators
JNL SOUZA and BH QUEIROGA substantially contributed to the design and planning, analysis and interpretation of the data and content, and the paper writing. PO KOCERGINSKY and PHC MARINHO significantly contributed in microbiological experiments, and in the paper writing. ÂT ARAKI significantly contributed in the paper preparation and writing.
REFERENCES
1. Dai T, Arce VJB, Tegos GP, Hamblin MR. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob Agents Chemother. 2011;55(12):5710-7. doi: 10.1128/AAC.05404-11 [ Links ]
2. Naglik JR, Paul L, Fidel PLJ, Odds FC. Animal models of mucosal Candida infection. National Institutes of Health. 2008;283(2):129- 9. doi: 10.1111/j.1574 6968.2008.01160.x
3. Lam M, Jou PC, Lattif AA, Lee Y, Malbasa CL, Mukherjee PK, et al. Photodynamic therapy with Pc 4 induces apoptosis of Candida albicans. Photochem Photobiol. 2011;87(4):904-9. doi: 10.1111/j.1751-1097.2011.00938.x
4. Kato TI, Prates RA, Sabino CP, Fuchs BB, Tegos GP, Mylonakis E, et al. Antimicrobial photodynamic inactivation inhibits Candida albicans virulence factors and reduces in vivo pathogenicity. Antimicrob Agents Chemother. 2013;57(1):445-51. doi: 10.1128/ AAC.01451-12
5. Kopnopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86(8):694-707. doi: 10.1177/154405910708600803
6. Teichert MC, Jones JW, Usacheya MN, Biel MA. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(2):155-60. doi: 10.1067/ moe.2002.120051
7. Pereira CA, Romeiro RL, Costa AC, Machado AK, Junqueira JC, Jorge AO. Susceptibility of Candida albicans, staphylococcus aureus, and streptococcus mutans biofilms to photodynamic inactivation: an in vitro study. Lasers Med Sci. 2011;26(3):341- 348. doi: 10.1007/s10103-010-0852-3.
8. Souza SC, Junqueira JC, Balducci I, Koga-ito CY, Munin E, Jorge AO. Photosensitization of different candida species by low power laser light. J Photochem Photobiol. B. 2006;83(1):34-8. doi:10.1016/j.jphotobiol.2005.12.002
9. Queiroga AS, Trajano VN, Lima EO, Ferreira AF, Limeira FA. J. In vitro photodynamic inactivation of candida spp. by different doses of low power laser light. Photodiagnosis Photodyn Ther. 2011;8(4):332-6. doi: 10.1016/j.pdpdt.2011.08.005.
10. Munin E, Giroldo LM, Alves LP, Costa MS. Study of germ tube formation by candida albicans after photodynamic antimicrobial chemotherapy (PACT). J Photochem Photobiol. B. 2007;88(1):16- 20. doi:10.1016/j.jphotobiol.2007.04.011
11. Donnely RF, Mccarron PA, Tunney MM. Antifungal photodynamic therapy. Res Microbiol. 2008;163(1):1-12. doi: 10.1016/j. micres.2007.08.001
12. Dovigo LN, Pavarina AC, Carmello JC, Machado AL, Brunetti IL, Bagnato VS. Susceptibility of clinical isolates of candida to photodynamic effects of curcumin. Lasers Surg Med. 2011;43(9):927-34. doi: 10.1002/lsm.21110
13. Kharkwal GB, Sharma SK, Huang YY, Dai T, Hanblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43(7):755-67. doi: 10.1002/lsm.21080
14. Pereira FG, Maisch T. Photodynamic inactivation to control infections caused by Candida albicans. Fungal Biol. 2012;116(1);1-10. doi: 10.1128/AAC.01451-12
15. Ryskova L, Buchta V, Slezak R. Photodynamic antimicrobial therapy. Cent Eur J Biol. 2010;5(4):400-406. doi: 102478/ s11535-010-0032-2
16. Dai T, Fuchs BB, Coleman JJ, Prates RA, Astrakas C, Denis TG, et al. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front Microbiol. 2012;3(120):1-16. doi: 10.3389/fmicb.2012.00120
17. Silva Martins J, Junqueira JC, Faria RL, Santiago NF, Rossoni RD, Colombo CE, et al. Antimicrobial photodynamic therapy in rat experimental candidiasis: evaluation of pathogenicity factors of candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(1):71-7. doi: 10.1016/j.tripleo.2010.08.012
18. Huang L, Xuan Y, Koide Y, Zhiyebtayev T, Tanaka M, Hamblin MR. Type I and type II mechanisms of antimicrobial photodynamic therapy: an in vitro study on gram-negative and gram-positive bacteria. Lasers Surg Med. 2012;44(6):490-9. doi: 10.1002/ lsm.22045
19. Mang TS, Mikulski L, Sala RE. Photodynamic inactivation of normal and antifungal resistant candida species. Photodiagnosis Photodyn Ther. 2010;7(2):98-105. doi: 10.1016/j. pdpdt.2010.03.001
20. Lyon JP, Moreira LM, Moraes PC, Santos FV, Resende MA. Photodynamic therapy for pathogenic fungi. Mycoses. 2011;54(5):265-71. doi: 10.1111/j.1439-0507.2010.01966.x
21. Chien HF, Chen CP, Chen YC, Chang PH, Tsai T, Chen CY. The use of chitosan to enhance photodynamic inactivation against candida albicans and its drug-resistant clinical isolates. Int J Mol Sei. 2013;14(4):7.445-7.456. doi: 10.3390/ijms14047445
22. Giroldo LM, Felipe MP, Oliveira MA, Munin E, Alves LP, Costa MS. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in candida albicans. Lasers Med Sci. 2009;24(1):109-12.
23. Souza RC, Junqueira JC, Rossoni RD, Pereira CA, Munin E, Jorge AO. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against candida albicans. Lasers Med Sci. 2010;25(3):385-9. doi: 10.1007/s10103-009-0706-z
 Correspondence to:
Correspondence to:
JNL SOUSA
e-mail: jnlopesodonto@gmail.com
Received on: 17/3/2015
Final version resubmitted on: 27/4/2015
Approved on: 11/5/2015













