Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.8 no.3 Joinville Jul./Set. 2011
ORIGINAL RESEARCH ARTICLE
Accuracy of oral exfoliative cytology in Sudanese patients undergoing oral biopsy
Ali Mahmoud M. EdrisI; Hussain Gadelkarim AhmedI; Elneel Ahmed MohammedII
I Department of Histopathology and Cytology, Faculty of Medical Laboratory Sciences – Khartoum – Sudan
II Faculty of Dentistry, University of Khartoum – Sudan
ABSTRACT
Introduction: Early detection of a premalignant or malignant oral lesions promises to improve the survival and the morbidity of patients suffering from these conditions. Oral exfoliative cytology (OEC) is a non-invasive method that is well accepted by the patient, and is therefore, suitable for screening at-risk population for early diagnosis of oral cancer. Objective: The purpose of this study is to investigate the value of OEC in the detection of oral premalignant and malignant lesions. Material and methods: In this hospital-based case-control study, cytological scrapes from buccal mucosa were obtained from 100 individuals, of whom 50 were patients with oral lesions ascertained as "cases" and 50 were clinically healthy volunteers ascertained as "controls". All patients with oral lesions were also subjected to oral biopsy and histological examination. Results: Out of 50 cases studied, histopathology showed the presence of: Oral squamous cell carcinoma OSCC (n = 28), leukoplakia (n = 8), dysplasia (n = 3), and benign normal lesions (n = 11). In cytology, a specificity of 100%, sensitivity of 93% and accuracy of 92% were obtained for OSCC. Leukoplakia gave a specificity of 100%, a sensitivity of 87.5%, and an accuracy of 95%. Conclusion: Despite the small number of cases in this study, OEC is a useful method for detecting oral premalignant and malignant lesions. OEC can detect a number of pathological conditions that require management.
Keywords: oral lesions; exfoliative cytology; diagnosis.
Introduction
Oral cancer is one of the most common malignancies as well as a major cause of cancer morbidity and mortality, worldwide 21. Most oral cancers are squamous cell carcinomas, and the vast majority of oral squamous cell carcinomas are preceded by precursor lesions that can be present as leukoplakia, erythroplakia, or erythroleukoplakia 14,17. Microscopically, these lesions may exhibit oral epithelial dysplasia, a histopathologic diagnosis characterized by cellular changes and maturational disturbances indicative of developing malignancy 22. Oral cancer is one of the major health problems in the Sudan, due to the habit of use of Toombak, which is known to contain high level of the potent carcinogenic component of the Tobacco Specific Nitrosamine (TSN) 11. The risk for cancer of the oral cavity among Toombak users was high (RR 7.3-73.0-fold) 9. Early detection of a premalignant or cancerous oral lesion promises to improve the survival and the morbidity of patients suffering from these conditions. Oral Exfoliative Cytology (OEC) is a non-aggressive technique that is well accepted by the patient, and is therefore an attractive option for the early diagnosis of oral cancer, including epithelial atypia and squamous cell carcinoma 5,15. In a study from Sudan, oral scrape smear cytological analysis has been proposed as a useful early diagnostic method for epithelial atypia and therefore also for malignant oral lesions 1. Despite the improvements in the methods used for collecting oral cytological material this methodology still presents problems in diagnosing oral cancer. Problems are mainly due to the existence of false negatives obtained as a result of a non representative sample as well as the subjectivity of the cytological evaluation 19. The purpose of this study was to evaluate the atypical cellular changes, as predictors for oral precancerous and cancerous lesions by means of exfoliative cytology assessment and to assess the correlation of cytological findings of the oral lesions to the clinically normal oral mucosal epithelium.
Material and methods
In this hospital-based case-control study, cytological scrapes of buccal mucosa were obtained from 100 individuals, of whom 50 were patients with oral lesions ascertained as "cases" and 50 were clinically healthy volunteers ascertained as "controls". All patients with oral lesions were also subjected to oral biopsy and histological examination. Two specimen types (biopsy or cytological smear) were obtained from each case; hence, only one specimen type (cytological specimen) was taken from each control. All participants were asked to sign a written consent form before taking the specimen.
Biopsy
A biopsy was taken from each patient with oral lesion after the surgical operation, then placed in 10% buffered formalin and sent to the laboratory for histopathology.
Biopsies with 5-mm diameter thickness were selected for tissues required for histopathology. These biopsies were processed in tissue processing machine up to the paraffin wax embedded blocks were prepared. Sections of 5 μm thickness were obtained from formalin-fixed paraffin wax embedded tissues using a rotary microtome.
Sections were stained using Hematoxylin and Eosin adopting Mayer's procedure.
Cytological smear
Using a flat wooden tongue spatula, cytological smears were collected from all patients without apparent oral lesions. The surface epithelium was scraped and cells were collected, immediately smeared on a cleaned frosted end glass slide, fixed in 95% ethanol for 15 minutes and then transferred to the laboratory at the Faculty of Medical Laboratory Science, University of Khartoum for further processing. Smears were further treated according to Papanicolaou method. Smears were hydrated in descending ethanol concentrations of 95% through 70% to distilled water, 2 minutes in each. For nuclear staining, smears were treated with Harris's Haematoxylin for 5 minutes, rinsed in distilled water and differentiated in 0.5% aqueous hydrochloric acid for 10 seconds to remove excess stain particles and then immediately rinsed in distilled water to stop decoloration. Thereafter, smears were stained blue in alkaline water for 4 seconds and dehydrated in ascending ethanol concentrations of 70% through two changes of 95%, 2 minutes in each. For the cytoplasmic staining, smears were treated with Papanicolaou Orange G6 solution for 2 minutes, rinsed in 95% ethanol and treated in Papanicolaou EA50 staining solution for 3 minutes. Finally, the smears were dehydrated in 95% through absolute ethanol, cleared in Xylene and then mounted in the DPX (Distrene Polystyrene Xylene) mount.
The assessment of cytological atypia was done using criteria described by Ahmed et al. 1. The presence of two or more of the following features indicates cytological atypia: nuclear enlargement associated with increased nuclear cytoplasmic ratio, hyperchromatism, chromatin clumping with moderately prominent nucleolation and irregular nuclear borders, bi or multinucleation, increase keratinization and scantiness of the cytoplasm and variations in size and/or shape of cells and nuclei.
Results
In this hospital-based case-control study a total of 100 individuals were studied, their ages ranging from 18 to 78, with a mean age of 45 years. The female male ratio was 1:1.3. All smears taken from the clinically healthy controls (n = 50) were found normal by cytology. Of the 50 cases, OSCC (n = 28), Leukoplakia (n = 8), dysplasia (n = 3) and Normal change (n = 11) were diagnosed by histopathology. In cytologically and histopathologically positive cases, cytology confirmed the histopathological diagnosis of OSCC in 27/28; all negative cases by histopathology were found negative in cytology. Of the 8 patients diagnosed as having leukoplakia by histopathology, seven were confirmed as leukoplakia by cytology. The three cases of dysplasia in histopathology were detected with dyskaryosis in cytology.
Of the 50 patients, 32 (64%) were males and 18 (36%) were females. Out of 39 (100%) neoplastic conditions, 27 (69%) were diagnosed in males.
The vast majority of the OSCCs were found in the lips followed by tongue, buccal mucosa, constituting 8 (28.5%), 7 (25%), 4 (14.3%) respectively, as shown in table I. Leukoplakia cases were increasingly identified in gingiva, tongue and buccal mucosa, constituting, 4 (50%), 2 (25%), 2 (25%), respectively. Two of the cases of dysplasia were found on buccal mucosa and the remaining one was found on the gingival aspects. Features of bacterial infection, fungal infection and inflammatory infiltrate were found in 20 (40%), 1 (2%), and 17 (34%) of the cases correspondingly, hence, such factors were identified in 3 (6%), 1 (2%) and 9 (18%) of the controls respectively.
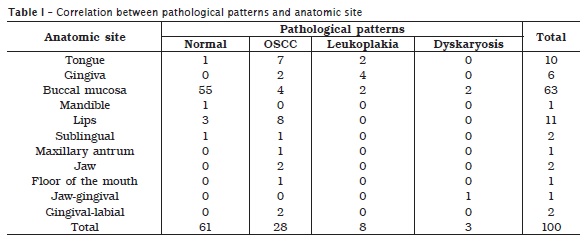
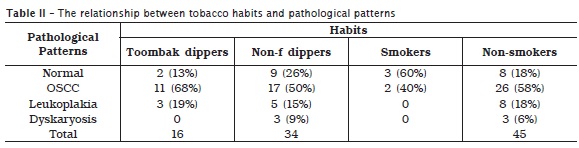
Eleven (39.3%) of the cases of OSCC and 3 (37.5%) of the cases of leukoplakia were found to have strong history of Toombak dipping (Local made smokeless tobacco). Moreover, two patients of the total five patients with a history of cigarette smoking were diagnosed with OSCC, as indicated in table II. These 13 cases of OSCC and the three of leukoplakia associated with tobacco use were revealed as habit practice for more than ten years. Furthermore, all those cases were found to dip Toombak or smoke cigarette for a frequency of more than 10 times or 10 cigarettes per day.
Discussion
Oral cancer is the most common cancer and constitutes a major health problem in developing countries, representing the leading cause of death 16. Accordingly, in this study, the frequency of oral cancer among patients with oral lesions is very high. In the Sudan, snuff, locally known as Toombak, was introduced approximately 400 years ago. The use of Toombak plays a significant role in etiology of oral squamous cell carcinomas (OSCCs), as it contains high level of the unusual potent carcinogenic component of the tobacco (TSN) 6,7,12. All of the cases of oral cancer in this study were defined as OSCC. Notably, many studies have reported a strong association between Toombak use and OSCC 4,8,10. Accordingly, the majority of OSCC lesions were found in lips and tongue, but variable intraoral site for OSCC have been extensively reported 13,18,24. Males represent the great majority of patients with oral lesions in the current study. However, it was well established that oral cancer is gender exclusive in the Sudan, due to the fact that Toombak use is uncommon among females, since it was considered as a social stigma in the Sudan 2.
Detection of high-risk oral premalignant lesions and intervention at premalignant stages might represent a success towards reducing the mortality and morbidity associated with OSCC. Histological examination of tissue remains the gold standard for diagnosis and identification of malignant oral lesions. Biopsy is an invasive technique with surgical implications, technique limitations for professionals and psychological implications for most patients. Consequently, exfoliative cytology has gained importance as a rapid and simple method. It is well known that diagnostic oral exfoliative cytology, despite being a useful, cost-effective and convenient tool in the diagnosis of oral precancerous and cancerous lesions, is not yet applied as widely as cervical cytology. Despite the small number of cases in this study, we think that, OEC has a reliable accuracy, and may be a useful screening tool for the diagnosis of oral premalignant and malignant lesions. Cytological scrapes from the clinically healthy volunteers were taken and labeled with those taken from the cases, in such a way that the investigator was not able to know the related link of the smear under examination. This is, however, added more reliability measures to our findings. As early diagnosis is of great importance for oral SCC, oral exfoliative cytology, a simple, painless and inexpensive method has become a preferred method for both early diagnosis of the lesion and for establishing quantitative techniques 20. The most challenging lesions of the oral cavity are the dysplastic lesions which are clinically often diagnosed as leukoplakias.3 However, the highest specificity and sensitivity measures in this study suggest that, OEC can be the preferred method for screening of oral mucosal lesions. It was found that, 4.5% of clinically benign-appearing lesions have dysplastic or carcinomatous features 23. Thus, cytological screening of the patients, at high risk of oral neoplastic lesions and without any macroscopically apparent oral lesion, can be of tremendous importance.
Although there is a significant increase in the frequency of patients presenting oral lesions in the Sudan (most related to Toombak use), there is no study assessing the value of OEC in comparison to histopathology. However, there were two studies reported from the Sudan, applying OEC as a diagnostic or screening method for identification of cytological atypia. The first study assessed by OEC showed cytological atypical changes in apparently healthy individuals (Toombak users, cigarette smokers or Non-tobacco users). It has been proposed that, oral scrape smear cytological analysis is a useful early diagnostic method for epithelial atypia 1. The second study applied OEC for the diagnosis of oral cancer and only those diagnosed as having OSCC were confirmed by histopathology 2.
In conclusion, oral cancer is on the increase in our setting and therefore there is need for public awareness, screening of all at-risk people, and early detection of the lesion through implementation of a national oral screening program. OEC is an accurate, simple, rapid, less invasive and relatively painless method, and is therefore, well accepted by patients, suitable for population screening programs and for early diagnosis of suspect oral lesions.
Acknowledgements
We would like to thank to the members of the Department of oral Maxillofacial surgery at Khartoum Teaching Dental Hospital for their cooperation and assistance. We are very grateful to the people at the Department of Histopathology and Cytology, Faculty of Medical Laboratory Sciences, University of Khartoum for their technical help and assistance.
References
1. Ahmed HG, Idris AM, Ibrahim SO. Study of oral epithelial atypia among Sudanese tobacco users by exfoliative cytology. Anticancer Res. 2003 Mar-Apr;23(2C):1943-9. [ Links ]
2. Ahmed HG, Mahgoob RM. Impact of Toombak dipping in the etiology of oral cancer: gender-exclusive hazard in the Sudan. J Can Res Ther. 2007 Apr-Jun;3(2):127-30.
3. Chattopadhyay A, Ray JG, Caplan DJ. AgNOR count as objective marker for dysplastic features in oral leukoplakia. J Oral Pathol Med. 2002 Oct;31(9):512-7.
4. Elbeshir EI, Abeen HA, Idris AM, Abbas K. Snuff dipping and oral cancer in Sudan: a retrospective study. Br J Oral Maxillofac Surg. 1989 Jun;27(3):243-8.
5. Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. J Can Dent. 2002 Nov;68(10):617-21.
6. Ibrahim SO, Lillehaug JR, Dolphine O, Johnson NW, Warnakulasuriya KA, Vasstrand EN. Mutations of the cell cycle arrest gene p21WAF1, but not the metastasis-inducing gene S100A4, are frequent in oral squamous cell carcinomas from Sudanese toombak dippers and non-snuff-dippers from the Sudan, Scandinavia, USA and UK. Anticancer Res. 2002 May-Jun;22(3):1445-51.
7. Ibrahim SO, Vasstrand EN, Johannessen AC, Idris AM, Magnusson B, Nilsen R et al. Mutations of the p53 gene in oral squamous-cell carcinomas from Sudanese dippers of nitrosamine-rich toombak and non-snuff-dippers from the Sudan and Scandinavia. Int J Cancer. 1999 May;81(4):527-34.
8. Idris AM, Ahmed HM, Mukhtar BI, Gadir AF, El-Beshir EI. Descriptive epidemiology of oral neoplasms in Sudan 1970-1985 and the role of toombak. Int J Cancer. 1995 Apr;61(2):155-8.
9. Idris AM, Ibrahim SO, Vasstrand EN, Johannessen AC, Lillehaug JR, Magnusson B et al. The Swedish snus and the Sudanese toombak: are they different? Oral Oncol. 1998 Nov;34(6):558-66.
10. Idris AM, Prokopczyk B, Hoffmann D. Toombak: a major risk factor for cancer of the oral cavity in Sudan. Prev Med. 1994 Nov;23(6):832-9.
11. Idris AM, Warnakulasuriya KA, Ibrahim YE, Nielsen R, Cooper D, Johnson NW. Toombak-associated oral mucosal lesions in Sudanese show a low prevalence of epithelial dysplasia. J Oral Pathol Med. 1996 May;25(5):239-44.
12. Loro LL, Vintermyr OK, Ibrahim SO, Idris AM, Johannessen AC. Apoptosis and expression of Bax and Bcl-2 in snuff- and non-snuff associated oral squamous cell carcinomas. Anticancer Res. 2000 Sep-Oct;20(5A):2855-60.
13. Mashberg A, Barsa P. Screening for oral and oropharyngeal squamous carcinomas. CA Cancer J Clin. 1984 Sep-Oct;34(5):262-8.
14. Mayne ST, Morse DE, Winn DM. Cancers of the oral cavity and pharynx. In: Schottenfeld D, Fraumeni Jr. JF, editors. Cancer epidemiology and prevention. New York: Oxford University Press; 2006. p. 674-96.
15. Mehrotra R, Gupta A, Singh M, Ibrahim R. Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions. Molecular Cancer. 2006 Mar;23:5-11.
16. Mehrotra R, Singh M, Kumar D, Pandey AN, Gupta RK, Sinha US. Age specific incidence rate and pathological spectrum or oral cancer in Allahabad. Ind J Med Sci. 2003 Sep;57(9):400-4.
17. Melrose RJ. Premalignant oral mucosal diseases. J Calif Dent Assoc. 2001 Aug;29(8):593-600.
18. Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and maxillofacial pathology. Philadelphia: W. B. Saunders Company; 1995. p. 297-301.
19. Nichols ML, Quinn FB Jr, Schnadig VJ, Zaharopoulos P, Hokanson JA, Des Jardins L et al. Interobserver variability in the interpretation of brush cytologic studies from head and neck lesions. Arch Otolaryngol Head Neck Surg. 1991 Dec;117(12):1350-5.
20. Paiva RL, Sant'Ana Filho M, Bohrer PL, Lauxen Ida S, Rados PV. AgNOR quantification in cells of normal oral mucosa exposed to smoking and alcohol: a cytopathologic study. Anal Quant Cytol Histol. 2004 Jun;26(3):175-80.
21. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74-108. 22. Pindborg JJ, Reichart PA, Smith CJ, van der Waal I. Histological typing of cancer and precancer of the oral mucosa. New York: Springer; 1997.
23. Sciubba JJ. Oral precancer and cancer: etiology, clinical presentation, diagnosis, and management. Compend Contin Educ Dent. 2000 Oct;21(10A):892-8.
24. Wood NK, Goas PW, editors. Diagnostico differencial de las lesions orales y maxilofacials. Madrid: Harcout Brace; 1998. p. 587-9.
 Correspondence:
Correspondence:
Ali Mahmoud M. Edris
University of Khartoum, 102, Faculty of Medical Laboratory Sciences
Khartoum – Sudan
E-mail: ali_edris74@yahoo.com
Received for publication: August 17, 2010
Accepted for publication: October 29, 2010













