Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.8 no.3 Joinville Jul./Set. 2011
ORIGINAL RESEARCH ARTICLE
Assessment of plasma and salivary antioxidant status in patients with recurrent aphthous stomatitis
Sudhanshu SaxenaI
I M.D.S., Senior Lecturer, Department of Public Health Dentistry, People's College of Dental Sciences and Research centre – Bhopal – Madhya Pradesh – India
ABSTRACT
Objective: The purpose of this study was to evaluate the antioxidant levels in plasma and saliva of patients with recurrent aphthous stomatitis (RAS) and healthy controls. Material and methods: Forty patients with RAS and 40 health controls were included into the study. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSHPx) activities and uric acid (UA) levels were measured in plasma and saliva. Chi-square and Student's t-test was used to estimate the significance between parameters. P value less than 0.05 was considered statistically significant. Results: Plasma analysis showed significantly decreased SOD and CAT activities, and UA level in RAS patients compared to control group. Plasma GSHPx activity was significantly higher in RAS patients. In saliva, SOD and CAT activities, and UA levels were significantly higher in RAS patients, while GSHPx activity was lower compared to control. Conclusion: Plasma and saliva antioxidant system is affected in RAS patients and both may be considered as an appropriate indicator of antioxidant status of body.
Keywords: recurrent aphthous stomatitis; antioxidants; saliva.
Introduction
Recurrent aphthous stomatitis (RAS) is one of the most common oral mucosal diseases. Clinical picture of RAS is defined by small, shallow, round or ovoid ulceration, with well defined and circumscribed margins and with an erythematous halo around it. Ulceration may be single or multiple. It has three clinical variants, of a minor, major and herpetiform RAS 2,10.
The exact etiology of RAS still remains unknown. No principal cause has been discovered, however, various factors such as local trauma, smoking, vitamin deficiency (folate, Vitamin B12, thiamine), virus (human herpes virus 6, human cytomegalovirus, Varicella zoster virus), bacteria (Streptococcus sanguis), hormones, genetics, nutrition, drug allergy, immunology may contribute to the pathogenesis of this clinical entity 6,13.
All of the above mentioned conditions can disturb the oxidant-antioxidant balance of the organism and can trigger the formation of free radicals. Oxidative stress occurs when the intracellular concentration of free radicals increases over the physiologic value. Free reactive oxygen radicals cause damage of mammalian cells by oxidizing fatty acids, protein and DNA. Also oxidative stress suppresses the immune system's ability to protect and restore affected cells 10,12.
To counteract the oxidative stress mammalian cells have antioxidant system which includes enzymatic activities such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSHPx), and non enzymatic antioxidants including vitamins A, E, C, melatonin, uric acid (UA) and glutathione 6,10.
Saliva is an important defense system of body. Recently studies have shown that saliva has its own antioxidant system including UA, SOD, GSHPx and CAT. UA constitutes around 70% of total antioxidant capacity of saliva 6,11.
However, there is paucity of research concerning plasma and salivary antioxidant levels with RAS. Hence, this present study was undertaken to evaluate the antioxidant levels in plasma and saliva of patients with recurrent aphthous stomatitis and healthy controls.
Material and methods
Selection of patients and control
Subjects of either gender attending the People's College of Dental Sciences and Research Centre, Bhopal, Madhya Pradesh, India with active episodes of RAS as well as healthy controls were included in the study groups.
The patients were selected based on the following criteria:
Inclusion criteria:
1) Subjects clinically diagnosed with recurrent aphthous stomatitis were included in the study group (RAS patient group).
2) Healthy subjects without RAS were included in the control group.
Clinical criteria considered to diagnose RAS:
RAS is essentially diagnosed by exclusion of other diseases. It occurs most commonly on buccal and labial mucosa. Lesions are less common on the heavily keratinized palate and gingiva. Localized burning or pain can be seen as a prodromal symptom 24-48 hours prior to classical clinical appearance. During this initial period, localized area of erythema develops. Within hours, a small white papule forms, ulcerates, and gradually enlarges over the next 48 to 72 hours. The individual lesions are round and symmetric but no tissue tags are present from the ruptured vesicles. Multiple lesions are often present. Lesions are typically very painful hence interferes with eating for several days 14.
In present study RAS was diagnosed with the help of an expert in oral medicine following the above mentioned criteria.
Exclusion criteria:
1) Subjects receiving any therapeutic regimen during the past 3 months.
2) Subjects receiving drugs containing iron and / or vitamins.
3) Subjects with Behcet's disease, chronic diarrhea, trauma history, and any other systemic disease.
4) Subjects with periodontal problems.
5) Subjects with history of smoking and alcohol.
On the basis of above mentioned criteria 40 patients diagnosed as RAS and 40 healthy controls without history of RAS episodes were included in the study. RAS patients and controls were matched for age and gender.
All the participants were explained about the need for undergoing a thorough clinical examination, blood and saliva investigations at the start of the study. Ethical clearance for the study was obtained from the Ethical Committee of the institution (protocol no. PCDS/Acad/Ethic/2010-11/79). Only those patients, who gave a signed informed consent on an approved proforma, were participated in the study.
Plasma preparation
Fasting venous blood samples (5ml) from RAS patients and control individuals were drawn into vacutainers containing heparin as anticoagulant. Blood was centrifuged at 1000 g for 10 min at 4ºC to obtain plasma, which was stored in small aliquots at –20ºC.
Saliva preparation
Unstimulated saliva samples were obtained following an overnight fast. Subjects were first asked to rinse their mouth using distilled water. Then they were told to sit comfortably. Collection of saliva was started after five minutes. Subjects were asked to spit into plastic tubes five times per minute for five minute. Samples were centrifuged 4000 g for 10 min at 4ºC; the upper parts were drawn and stored in small aliquots at –20ºC.
Protein determination
Protein measurements were made according to method described by Lowry et al. 8.
Assay of GSHPx activity
The GSHPx activity was measured according to Lawrence and Burk 7. One ml of 50 μM phosphate buffered saline (PBS) solution (pH 7.4) including 5 μM ethylene diamine tetraacetic acid (EDTA), 2 μM nicotinamide adenine dinucleotide phosphate (NADPH), 20 μM Glutathione (GSH), 10 μM NaN3 and 23 mU of oxidized glutathione reductase (GSSG-R) was incubated at 37ºC for 5 min. Then 20 μl of 0.25 μM H2O2 solution and 20 μl sample (plasma or saliva) were added to assay. The change in the absorbance at 340 nm was monitored for 1 min by spectrophotometer. A blank with all ingredients except sample was also monitored. Specific activity was calculated as μM of NADPH consumed per minute per milligram of protein (U/mg protein) using appropriate molar absorption coefficient (ε = 6220).
Assay of SOD activity
The SOD activity was measured according to McCord and Fridovich 9. Solution A was prepared by mixing 100 ml of 50 mM PBS (pH 7.4) including 0.1 mM EDTA and 2 μM cytochrome c with 10 ml 0.001 N NaOH solution including 5 μM xanthine. Solution B was prepared by mixing 0.2 U xanthine oxidase/ml and 0.1mM EDTA. Fifty microliters of sample (plasma or saliva) were mixed with 2.9 ml of solution A followed by 50 μl of solution B. Change in absorbance was measured at 550 nm. A blank was run with fifty microliters of ultra pure water. SOD levels were expressed as U/mg protein with reference to the activity of a standard sample of bovine copper-zinc SOD under the same conditions.
Assay of CAT activity
The CAT activity was measured in samples by method applied by Cimen et al. 4 Decomposition of H2O2 was monitored at 240 nm by spectrophotometer. Specific activity was determined as micromole of substrate H2O2 decomposed per minute per milligram of protein (U/mg protein).
Determination of uric acid concentration
Method Proposed by Fossati et al. was used to measure UA concentration 5. UA was transformed by uricase into allantoin and H2O2, which under the catalytic influence of peroxidase, oxidized to the chromogen (4-aminophenazone/N-ethyl-methylaniline propanesulfonate sodic) to form a red compound whose intensity of color was proportional to the amount of UA present in sample. Change in absorbance was measured at 546 nm and finally UA levels were expressed as mg/dl.
Statistical analysis
All values were expressed as mean ± standard deviation (SD). Chi-square and Student's t-test were used to estimate the significance between parameters. P value less than 0.05 was considered statistically significant.
Results
The mean ± SD age of RAS patients and control group were 24.62 ± 7.25 and 24.72 ± 7.20 years respectively (p > 0.05). The patients group comprised of 18 males and 22 females, while control group consisted of 20 males and 20 females (p > 0.05).
Analysis of antioxidants in plasma showed significantly decreased SOD and CAT activities, and UA level in RAS patients compared to control group. Plasma GSHPx activity was significantly higher in RAS patients (table I).
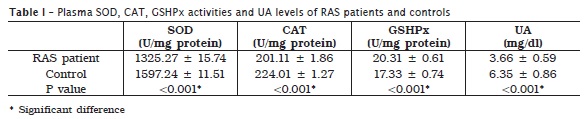
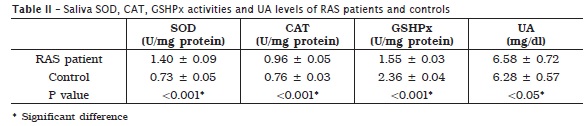
In saliva, SOD and CAT activities, and UA levels were significantly higher in RAS patients. At the same time RAS patients showed significantly lower activity of GSHPx in saliva, compared to control group (table II).
Discussion
In this present study, the author investigated whether there is any alteration in SOD, GSHPx, CAT activities and UA levels in plasma and saliva of RAS patients when compared to those of the control group.
Whole and unstimulated saliva was used to assess antioxidant parameters. Whole saliva is the most relevant, as it reflects more closely the predominant intra-oral condition. Stimulation may result in false increase in the concentration of antioxidants in saliva due to increase in flow of gingival crevicular fluid 3.
Analysis of antioxidants in plasma showed significantly decreased SOD and CAT activities and UA level and increased GSHPx activity in RAS patients. Results from saliva samples of RAS patients were totally opposite of antioxidant composition in plasma.
Karincaoglu et al. 6 evaluated plasma and saliva antioxidant activity in patients with RAS and reported that SOD and CAT activity in plasma of RAS patients was lower than of control group. At the same time there was an increase in GSHPx activity in plasma of RAS patients. Reversed results were observed in saliva antioxidant profile. Difference between salivary UA levels of RAS patients and control was not significant. Cimen et al. 9 measured SOD, GSHPx, and CAT activity in plasma of RAS patients and control group. They observed a relative reduction in CAT and GSHPx activity. Studies conducted by Momen-Beitollahi et al. 10 and Caglayan et al. 3 showed no difference in plasma and saliva total antioxidant status between RAS patients and control. These relative controversies among studies may be associated with several factors including sample size variations, application of various methods and genetic divergence of each population. Furthermore, the composition and antioxidant capacity may vary by dietary and nutrient deficiency 10. There is need to study these parameters with respect to RAS.
SOD is an enzyme that catalyzes the dismutation of two superoxide anions (O2-) into hydrogen peroxide and molecular oxygen. GSHPx is the dominant antioxidant protective element participating in getting rid of H2O2 in the site by consumption of reduced GSH. CAT recognized to be secondary antioxidant enzyme in peroxidative defense. It hydrolyzes H2O2 into H2O and O2 6,10. Uric Acid represents a final product in the metabolism of the purines, acting as a potent free radical scavenger and inhibitor of lipid peroxidation 1.
As the SOD activity is high in saliva but low in plasma, it lead to bear in mind that antioxidant molecules are likely to be transferred from plasma to the areas where ulcers occurs. Reason could be the same for CAT activity and UA levels. Increased SOD activity in saliva resulted in overproduction of H2O2. During the detoxification of increased H2O2 by GSHPx, consumption of reduced GSH also increases. Because of enough unprovided GSH, the activity of GSHPx may have decreased in saliva of RAS patients. Limited diffusion of H2O2 from lesion resulted in increased activity of GSHPx in plasma as feedback effect of H2O2 on mRNA expression 6. However, molecular studies on the subject are needed to prove or refute the above mentioned hypothesis.
Conclusion
Plasma and saliva antioxidant system is affected in RAS patients and both may be considered as an appropriate indicator of antioxidant status of body. However, the relationship between free radicals and RAS need to be studied extensively in many aspects.
Acknowledgements
I would like to acknowledge the valuable guidance of Professor Dr. Amrit Pal Kaur in the Bio-chemical analysis of plasma and saliva samples.
References
1. Altinkaynak K, Varoglu AO, Aksoy H, Deniz O, Aksoy A. Serum uric acid levels in patients with relapsing-remitting multiple sclerosis. Eur J Gen Med. 2009;6(3):166-9. [ Links ]
2. Altinyazar HC, Gurel A, Koca R, Armutcu F, Unalacak M. The status of oxidants and antioxidants in neutrophils of patients with recurrent aphthous stomatitis. Turk J Med Sci. 2006;36:87-91.
3. Caglayan F, Miloglu O, Altun O, Erel O, Yilmaz AB. Oxidative stress and myeloperoxidase levels in saliva of patients with recurrent aphthous stomatitis. Oral Dis. 2008 Nov;14(8):700-4.
4. Cimen MY, Kaya TI, Eskandari G, Tursen U, Ikizoglu G, Atik U. Oxidant/antioxidant status in patients with recurrent aphthous stomatitis. Clin Exp Dermatol. 2003 Nov;28(6):647-50.
5. Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzyme assay of uric acid in serum and urine. Clin Chem. 1980 Feb;26(2):227-31.
6. Karincaoglu Y, Batcioglu K, Erdem T, Esrefoglu M, Genc M. The levels of plasma and salivary antioxidants in the patient with recurrent aphthous stomatitis. J Oral Pathol Med. 2005 Jan:34(1):7-12.
7. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun. 1976 Aug;71(4):952-8.
8. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Biol Chem. 1951 Nov;193(1):265-75.
9. McCord JM, Fridovich I. Superoxide dismutase: an enzymatic function erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov;244(22):6049-55.
10. Momen-Beitollahi J, Mansourian A, Momen-Heravi F, Amanlou M, Obradov S, Sahebjamee M. Assessment of salivary and serum antioxidant status in patients with recurrent aphthous stomatitis. Med Oral Patol Oral Cir Bucal. 2010 Jul;15(4):e557-61.
11. Nagler RM, Klein J, Zarzhevsky N, Drigues N, Reznick A. Characterization of the undifferentiated antioxidant profile of human saliva. Free Radic Biol Med. 2002 Feb;32(3):268-77.
12. Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 1998;9(3):306-21.
13. Safadi RM. Prevalence of recurrent aphthous ulceration in Jordanian dental patients. BMC Oral Health. 2009 [cited 2010]. Available from: URL: http://www.biomedcentral.com/1472-6831/9/31.
14. Woo SB, Greenberg MS. Ulcerative, vesicular and bullous lesions. In: Greenberg MS, Glick M, Ship JA, editors. Burket's Oral Medicine. Hamilton: Decker Inc.; 2008. p. 57-60.
 Correspondence:
Correspondence:
Sudhanshu Saxena
Department of Public Health Dentistry – People's College of Dental
Sciences & Research Centre – People's group
Bhanpur, Bhopal
462037 – Madhya Pradesh – India
E-mail: dr.sudhanshusaxena@gmail.com
Received for publication: September 6, 2010
Accepted for publication: November 4, 2010













