Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.8 no.3 Joinville Jul./Set. 2011
ORIGINAL RESEARCH ARTICLE
Effects of catalase, 2% chlorhexidine gel and 1% sodium hypochlorite on the microtensile bond strength of teeth bleached with 35% hydrogen peroxide
Ricardo FerreiraI; Tassiana Vieira NunesII; Betsy Kilian Martins LuizIII; Rubens Nazareno GarciaI,IV
I School of Dentistry, University of Itajai Valley – Itajai – SC – Brazil
II DDS, Private Office
III School of Dentistry, Federal University of Santa Catarina – Florianopolis – SC – Brazil
IV School of Dentistry, University of Joinville Region – Joinville – SC – Brazil
ABSTRACT
Introduction and objective: Dental bleaching is an effective, relatively simple and noninvasive technique. The aim of this study was to evaluate the effects of catalase, 2% chlorhexidine gel, and 1% sodium hypochlorite on the microtensile bond strength to enamel of bovine teeth submitted to internal and external bleaching with 35% hydrogen peroxide. Material and methods: Sixty bovine incisors were used. They had their debris removed, washed in tap water and stored frozen. The samples were divided into five experimental groups according to the treatment applied after bleaching (n = 12): 1 − control/no bleaching (C); 2 − catalase (CA); 3 − 2% chlorhexidine gel (CG); 4 − 1% sodium hypochlorite (SH); 5 − distilled water (DW). For microtensile test, samples were prepared into blocks of enamel/resin, which were sectioned to obtain hourglass-like specimens. Bond strength was calculated in MPa and data analyzed statistically by Anova (p < 0.05). Results: Microtensile bond strength means decreased in comparison to control group, but no statistically significant difference between groups was found. Conclusion: The substances used after dental bleaching did not result in statistically significant microtensile bond strength means of the tested groups.
Keywords: dental enamel; tensile strength; tooth leaching.
Introduction
Among the several aesthetic dental treatments, bleaching has draw some attention by patients and dentists because represents a noninvasive, relatively simple procedure to be performed 9. This treatment success is related to bleaching material’s ability of penetrating into dentinal tubules. The deeper the penetration, the more the pigment that causes chromatic alteration can be reversed by the oxidation reaction, converting dark molecules into carbon dioxide and water 6.
Dental bleaching has been classified into two types. The first comprises the application of the bleaching agent and/or an energy source (light), generally used in vital teeth. The second uses the bleaching agent inside root canal, after the biomechanical preparation, so-called the walking bleach technique 10. In this technique, the bleaching agents are placed inside the root canal, directly in contact with dentin. The most two used agents are hydrogen peroxide and sodium perborate. Although these substances are efficient in altering tooth color, complications in dental bleaching have been reported, including alterations in dentin surface morphology, changes in dentin composition and permeability, and external root resorption 7,11.
Hydrogen peroxide (H2O2) is a largely employed, safe, and effective bleaching agent whose exact mechanism of bleaching action is not completely understood. One possible theory is that H2O2 is decomposed to produce oxygen radicals, which attack organic pigment molecules, causing the whitening effect 12.
According to Torneck et al. (1990) 22, the presence of residual peroxide after bleaching procedures may result in bond strength decreasing of esthetical restorations on enamel surface. Rotstein (1993) 15 reported that catalase application, for 3 minutes, would completely eliminate the residual H2O2.
Sodium hypochlorite is the chemical solution mostly used as adjuvant of root canal shaping. In addition to its bleaching property, sodium hypochlorite presents antimicrobial activity against bacteria, fungi, and virus; and, acts as a low surface tension organic solvent, deodorant, lubricant 14.
Chlorhexidine is a bactericide and bacteriostatic substance that inhibits the enzymatic activity and presents a relative lack of toxicity and odor. Also, it presents a broad spectrum antibacterial activity and substantivity, i.e., the ability of slow releasing as its concentration decreases, which enables a long action time period 14.
The aim of this study was to evaluate the effects of catalase, 2% chlorhexidine gel, and 1% sodium hypochlorite on the microtensile bond strength to enamel of bovine teeth, submitted to internal and external bleaching with 35% hydrogen peroxide.
Material and methods
Sixty bovine incisors were used and had their debris removed. Following, teeth were washed in tap water and kept frozen. Each sample was sectioned transversally at 10 mm of its crown portion and at 5 mm of its root portion, from enamel-cement junction, through double-face diamond discs (#7016, KG Sorensen), at low speed under water cooling.
Pulp was removed by dental probe number #5 (Duflex, SS White) and pulp chamber width was standardized as the active point of a carbide round bur number #8 (Fava), at high speed under water cooling. Teeth were stored in a humidifier chamber at 37ºC before and after the bleaching procedure.
35% hydrogen peroxide (Whiteness HP) was mixture according to the manufacturer’s instructions, using a bleaching agent/thickener ratio of 3:1. This was applied onto tooth’s labial surface and pulp chamber by using the application tips (approximately 1 mm of thickness). Then, the agent was light-cured for 30 seconds by a LED device (LED Radii Cal, SDI, 1200 mW/cm2). This procedure was repeated twice, totalizing 45 minutes (15-min interval for each application). All this process comprising three applications of the bleaching agent was repeated at every 7 days, during three weeks. After the last week, the samples were stored in a humidifier chamber at 37ºC, for 1 week.
Next, the samples were randomly divided into five test groups, according to the substance applied after dental bleaching (n = 12): 1 – control/no bleaching (C); 2 – catalase (CA); 3 – 2% chlorhexidine gel (CG); 4 – 1% sodium hypochlorite (SH); 5 – distilled water (DW).
Samples were cut, longitudinally at mesiodistal direction, under water cooling (Isomet, Buehler, Ltd., USA). This allowed the discard of the lingual surface and the exposure of the pulp chamber’s internal surface. Prior to the bond procedures, the aforementioned substances were applied according to each test group, for 5 minutes. Following, the samples were washed with distilled water for 1 minute and dried.
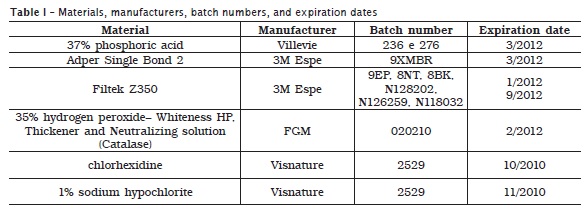
The outlined area of each sample (enamel fragment) was etched by 35% phosphoric acid, for 30 seconds, washed for 10 seconds and dried. Two consecutive layers of the adhesive system Adper Single Bond 2 were applied, gently air dried, and light-cured for 10 seconds. A composite resin (Filtek Z350) was placed at 2-mm increments, which were light-cured for 20 seconds each. Table I shows the materials employed, and their manufacturers, batch numbers, and expiration dates.
For microtensile bond strength tests, enamel/resin blocks were sectioned at about 1-mm cuts and prepared to obtain hourglass-like samples. Prior to this test, enamel/resin interface area were measured and calculated by using a digital caliper (Mahr 16ES, Carl Mahr, Germany). Samples were fixed into a device with cianoacrilate (Superbonder gel/Loctite) and microtensile bond strength test was performed in a universal test machine (Instron 4411, Instron Corp, USA), at 0.5 mm/min speed.
Microtensile bond strength was calculated and expressed in MPa and data were statistically analyzed by analysis of variance (Anova), with level of significance set at 5%.
Results
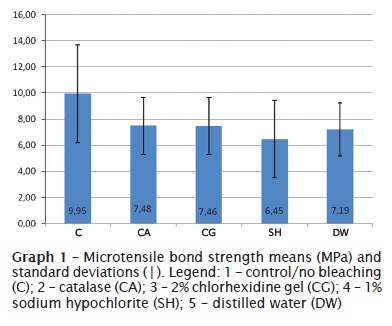
Anova did not show statistically significance differences at 5% of significance level. Microtensile bond strength mean values and standard deviation, for each group, are seen in graph 1.
Discussion
Dental aesthetics has been an issue of great interest, nowadays. Accordingly, dental bleaching has been widely studied because it is a noninvasive aesthetic treatment showing very successful results. This present study did not observe statistically significant differences among the microtensile bond strength means of control and bleaching groups receiving application of catalase, 2% chlorhexidine gel, 1% sodium hypochlorite, and distilled water. Amaral et al. (2008) 1 also did not find any reduction in bond strength to enamel after dental bleaching by carbamide peroxide. Bating et al. (2004) 4 concluded that carbamide peroxide at different concentrations did not present statistically significant differences in bond strength, after 15-day storage in saliva. Although this is relevant information, carbamide peroxide was not the agent used in this present study.
The findings of Teixeira et al. (2002) 20 stated that 7, 14, and 21 days after internal bleaching, the shear bond strength to bovine tooth’s enamel was not altered. However, shear bond strength values were affected by the mixture of sodium perborate and 30% hydrogen peroxide, immediately after dental bleaching. Following the same research line, Teixeira et al. (2004) 21 showed that bleaching procedures with 37% carbamide peroxide did not affect bond strength to enamel and dentin. Notwithstanding, these values decrease when sodium perborate was associated with 30% hydrogen peroxide, regardless of the time elapsed after the bleaching procedure.
Lewinsten et al. (1994) 13 reported that 30% hydrogen peroxide reduced enamel and dentin’s microhardness and this reduction was statistically significant after 5 and 15 minutes for dentin and enamel, respectively. Also, Borges et al. (2010) 5 concluded that bleaching acid gels reduced enamel’s microhardness and the use of remineralizing gels after the bleaching procedure may improve the microhardness values of the bleached enamel. In this present study, we did not use the dentin substrate and microhardness tests; however, microhardness data are relevant and largely studied concerning to bleaching agents.
On the other hand, the findings of Barcellos et al. (2010) 2 showed significant differences in bond strength values after 14 days of bleaching in enamel substrate groups. Moreover, the damages caused by bleaching agents in bond strength of resin restoration to tooth structure are increased as the concentration of these agents increase. Concerning to the groups in which the restoration was inserted onto enamel, the authors affirmed that the control group presented the highest bond strength, with statistically significant differences.
Barbosa et al. (2008) 3 recommended that bonding procedures to enamel be postponed until seven days post-bleaching treatment with 35% hydrogen peroxide, while restorations onto dentin should be postponed until 14 days after bleaching. Our study followed these authors’ recommendations. Accordingly, Shinohara et al. (2005) 16 stated that the decrease in shear bond strength values is time-dependent and, they recommended to wait longer periods for performing composite resin restorations.
Accordingly, a satisfactory period, for composite restorations both on enamel and dentin, would be two weeks post-bleaching.
Cavalli et al. (2001) 8 reported that, in the first two weeks after bleaching, the bond strength of composite resin to enamel was low. However, after three weeks, the bond strength values returned to those of the control group (without treatment). Torneck et al. (1990) 22 suspected that this decreased adhesive bond strength was caused by the presence of residual peroxide or peroxide-related substances at or near the enamel surface. Following this same line, Rotstein (1993) 15 reported that hydrogen peroxide at high concentrations may cause damages to tooth structure and periodontal tissues and, catalase may be efficiently applied after internal bleaching to eliminate the residual hydrogen.
Sung et al. (1999) 18 still affirmed, based on the results of their study, that it would be more adequate to use alcohol-based adhesives because they result in higher shear bond strength values. Therefore, composite resin restorations could be performed immediately after bleaching. Our study used the adhesive system Adper Single Bond 2, which presents alcohol as solvent. Despite this fact, based on literature, we opt to wait one week after bleaching to perform the resin insertion, for all groups.
In agreement with the findings of our study, Tamura et al. (2008) 19, using tensile bond strength tests similar to those employed in this present study, found no statistically significant tensile bond strength mean values after three applications of in-office bleaching, regardless the site of application, the type of the bleaching agent, and the type of light-curing.
Conclusion
Based on the obtained data and the applied statistical analysis, it can be concluded that the substances employed after bleaching procedures did not result in statistically significant microtensile bond strength mean values.
References
1. Amaral C, Jorge A, Veloso K, Rodrigues J, Erhardt M, Arias V. The effect of in-office in combination with intracoronal bleaching on enamel and dentin bond strength and dentin morphology. J Contemp Dent Pract. 2008 Jul;9(5):17-24. [ Links ]
2. Barcellos DC, Benetti P, Fernandes Junior VVB, Valera MC. Carbamide peroxide bleaching gel concentration on the bond strength of dental substrates and resin composite. Oper Dent. 2010;35(4):463-9.
3. Barbosa CM, Sasaki RT, Florio FM, Basting RT. Influence of time on bond strength after bleaching with 35% hydrogen peroxide. J Contemp Dent Pract. 2008;9(2):81-8.
4. Basting T, Rodrigues JA, Serra MC, Pimenta LAF. Shear bond strength of enamel treated with seven carbamide peroxide bleaching agents. J Esthet Restor Dent. 2004;6:250-60.
5. Borges AB, Yui KC, D’Avila TC, Takahashi CL, Torres CR, Borges AL. Influence of remineralizing gels on bleached enamel microhardness in different time intervals. Oper Dent. 2010 Mar-Apr;35(2):180-6.
6. Carrasco LD, Guerisoli DMZ, Rocha MJA, Pécora JD, Froner IC. Efficacy of intracoronal bleaching techniques with different light activation sources. Int Endod J. 2007 Mar;40(3):204-8.
7. Carrasco LD, Guerisoli DMZ, Rocha MJA, Pécora JD, Froner IC. Evaluation of dentin permeability after light activated internal dental bleaching. Dent Traumatol. 2007 Feb;23(1):30-4.
8. Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001;26(6):597-602.
9. Faraoni-Romano JJ, Turssi CP, Serra MC. Effect of a 10% carbamide peroxide on wear resistance of enamel and dentine: in situ study. J Dent. 2009 Apr;37(4):273-8.
10. Hosoya N, Cox CF, Arai T, Nakamura J. The walking bleach procedure: an in vitro study to measure microleakage of five temporary sealing agents. J Endod. 2000 Dec;26(12):716-8.
11. Korkmaz Y, Onay EO, Ozel E, Ungor M. Sealing capacity of a flowable composite, as a protective base, with different conditioning methods in nonvital bleaching. Photomed Laser Surg. 2008;26(4):355-9.
12. Lee HW, Kim GJ, Kim JM, Park JK, Lee JK, Kim GC. Tooth bleaching with nonthermal atmospheric pressure plasma. J Endod. 2009 Apr;35(4):587-91.
13. Lewinsten I, Hirschfeld Z, Stabholz A, Rotstein I. Effect of hydrogen peroxide and sodium perborate on the microhardness of human enamel and dentin. J Endod. 1994;20(2):61-3.
14. Lopes HP, Siqueira Júnior JF. Endodontia: biologia e técnica. Guanabara Koogan: Rio de Janeiro; 2004.
15. Rotstein I. Role of catalase in the elimination of residual hydrogen peroxide following tooth bleaching. J Endod. 1993;(19):567-9.
16. Shinohara MS, Peris AR, Pimenta LA, Ambrosano GM. Shear bond strength evaluation of composite resin on enamel and dentin after nonvital bleaching. J Esthet Rest Dent. 2005;17(1):22-9.
17. Stokes AN, Hood JAA, Dhariwal D, Patel K. Effect of peroxide bleaches on resin-enamel bonds. Quintessence Int. 1992;23(11):769-72.
18. Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite resin to dental bonding agent enhanced enamel. J Prosthet Dent. 1999;82:595-9.
19. Tamura T, Tonami K, Takahashi H, Mataki S, Araki K, Kurosaki N. Tensile strength of dentin after bleaching treatment. J Med Dent Sci. 2008 Mar;55(1):175-80.
20. Teixeira ECN, Hara AT, Turssi CP, Serra MC. Effect of nonvital tooth bleaching on resin/enamel shear bond strength. J Adhes Dent. 2002;(4):317-22.
21. Teixeira ECN, Turssi CP, Hara AT, Serra MC. Influence of post-bleaching time intervals on dentin bond strength. Restorative Dent. 2004;18(1):75-9.
22. Torneck GD, Titley KG, Smith DC, Adibfar A. The influence of time of hydrogen peroxide exposure on the adhesion of composite resin to bleached bovine enamel. J Endod. 1990;(16):123-8.
 Correspondence:
Correspondence:
Rubens Nazareno Garcia
Faculdade de Odontologia – Universidade do Vale do Itajaí
Rua Uruguai, n. 458.
CEP 88302-202 – Itajaí – SC – Brasil
E-mail: ali_edris74@yahoo.com
Received for publication: January 10, 2011
Accepted for publication: March 1, 2011













