Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.8 no.3 Joinville Jul./Set. 2011
ORIGINAL RESEARCH ARTICLE
Hydrogen ion and calcium releasing of MTA Fillapex® and MTA-based formulations
Milton Carlos KugaI; Edson Alves de CamposI; Paloma Hernandez ViscardiII; Paula Zapparoli CarrilhoII; Fernanda Castilho XaviérII; Nayara Pereira SilvestreII
I School of Dentistry of Araraquara, Sao Paulo State University – Araraquara – SP – Brazil
II School of Dentistry, University Center of Rio Preto – Sao Jose do Rio Preto – SP – Brazil
ABSTRACT
Introduction: MTA is composed of various metal oxides, calcium oxide and bismuth. It has good biological properties and is indicated in cases of endodontic complications. Several commercial formulations are available and further studies are necessary to evaluate these materials. Objective: To evaluate pH and calcium releasing of MTA Fillapex® compared with gray and white MTA. Material and methods: Gray and white MTA (Angelus) and MTA Fillapex® (Angelus) were manipulated and placed into polyethylene tubes and immersed in distilled water. The pH of these solutions was measured at 24 hours, 7 days and 14 days. Simultaneously, at these same aforementioned periods, these materials' calcium releasing was quantified, through atomic absorption spectrophotometry. The results were submitted to ANOVA, with level of significance at 5%. Results: Concerning to pH, the materials present similar behaviors among each other at 24 hours (p > 0.05). At 7 and 14 days, MTA Fillapex® provided significantly lower pH values than the other materials (p < 0.05). Regarding to calcium releasing, at 24 hours and 7 days, MTA Fillapex® provided lower releasing than the other materials (p < 0.05). After 14 days, differences were found between MTA Fillapex® and gray MTA (p < 0.05). Conclusion: All materials showed alkaline pH and calcium releasing, with significantly lower values for MTA Fillapex® sealer.
Keywords: MTA; pH; calcium; sealer.
Introduction
Mineral trioxide aggregate (MTA) was idealized to be employed in endodontic accidents and periradicular surgeries 20,26. It is constituted of a fine hydrophilic powder (SiO2, K2O, Al2O3, SO3, CaO and Bi2O3) almost always mix with distilled water 21. MTA is commercialized in two formulations: gray (GMTA) and white (WMTA). They are differentiated mostly with regards to their chemical composition, because gray MTA has a higher concentration of aluminum, magnesium, and mainly iron oxide 1. The biological results demonstrated that MTA provides favorable tissue response, characterized by lack of severe inflammation, presence of fibrous capsule, and induction of hard tissue 2,10.
Although MTA has several favorable properties supporting its clinical use, there are many problems that make handling difficult due to the granular consistency 15, long setting time 6, and possible displacement out of the cavity in which it was inserted 17. Most of endodontic complications are located at difficult access sites, inhibiting MTA employment. Therefore, the evaluation of periradicular tissues' biological response after root canal filling with MTA/distilled water and MTA/propyleneglycol was assessed. The results showed that the pastes presented a similar biological behavior, especially when they had been kept only within root canals 13. The authors highlighted that although the association between MTA and propyleneglycol is more easily placed into root canals, there is no setting.
On the other hand, MTA's physical characteristics after mixture make root canal filling difficult, since mixing MTA with distilled water produces a granulous mixtures 7. Currently, endodontic sealers for intracanal filling containing MTA have been developed, for example, Endo CPM Sealer (EGEO, Buenos Aires, Argentina), ProRoot Endo Sealer (Dentsply Maillefer, Ballaigues, Switzerland), and the experimental sealer MTAS (an association between 80% of white Portland cement and 20% of bismuth oxide) with and addition of water soluble polymers 5. Sealers containing MTA are biocompatible and stimulate mineralization 10. They also exhibit a higher adhesiveness to dentin than conventional zinc oxide/eugenol-based cements 14 and sealing ability similar to epoxy resin-based cements 28.
A MTA endodontic sealer (MTA Fillapex®, Angelus Soluções Odontológicas, Londrina, PR, Brazil) was recently created. According to the manufacturer, its composition after mixture is basically MTA, salicylate resin, natural resin, bismuth and silica. To date, there is a lack of studies evaluating the material, concerning to its physico-chemical and biological properties.
Ideally, a material should present alkaline pH and calcium realese to stimulate tissue mineralization 9,12. Therefore, the aim of this study was to evaluate the hydrogen potential and calcium releasing of MTA Fillapex® by comparison with gray and white MTA mixed to distilled water, at 24 hours, 7 and 14 days.
Material and methods
Sample preparation
Thirty polyethylene tubes, measuring 0.5 cm length x 1.0 mm internal diameter, was filled with the tested materials and divided into 3 groups (n = 10). In group I (G I) – control group – 1 g of gray MTA (Angelus Soluções Odontológicas, Londrina, PR, Brazil) was mixed with 0.30 ml of distilled water. This same procedure was used in Group II (G II) however employing white MTA (Angelus Soluções Odontológicas, Londrina, PR, Brazil). In Group III (G III), MTA Fillapex® (Angelus Soluções Odontológicas, Londrina, PR, Brazil) was used and proportioned according to its self-mixing system.
The polyethylene tubes were filled with the materials with aid of a Lentulo spiral (Maillefer, Ballaigues, Switzerland) immediately. The materials were weighed to allow a similar amount of material (+ 0.002 g) in each sample. After filling, the tubes were placed inside polypropylene flasks (Injeplast, Sao Paulo, SP, Brazil), containing 10 ml of distilled water, closed and stored in at constant temperature of 37ºC (Farmen, Sao Paulo, SP, Brazil) during all the evaluation period. At 24 hours, 7 and 14 days, pH and calcium releasing was measured in the water. Following each evaluation, the water was discarded, and the tubes were immersed in new flasks containing similar amounts of distilled water. This distilled water was previously tested and certified regarding to the absence of calcium ions and the presence of a neutral pH (6.8).
pH analysis
The water pH in which the polyethylene tubes were immersed was measured at 24 hours, 7 and 14 days. This was performed through a pH meter (DM22 model, Digimed, Sao Paulo, SP, Brazil) calibrated with standard solutions of pH 4.0 and 7.0, at constant temperature of 25ºC. At, 24 hours post-immersion, the tubes were carefully removed out of the flasks and immersed in new flasks containing similar amounts of new distilled water. This procedure was repeated until totalizing 336 hours, since the solution was replaced at 24 hours, 7 and 14 days. The obtained pH values were submitted to one-way analysis of variance and Tukey's test, with level of significance at 5%.
Calcium releasing analysis
Calcium ions releasing was measured through an atomic absorption spectrophotometer (AA6800, Shimadzu, Tokyo, Japan), equipped with a specific cathode lamp for reading. The conditions for the device use were determined according to the manufacturer's instructions: 422.70 nm wavelength, 2.01/min slot. To prevent a possible interference by other alkaline metals, a lanthanum solution was prepared by diluting 9.8 g of lanthanum chloride in 250 ml of acid solution. This was posteriorly added to the water in which the polyethylene tubes were immersed. By diluting calcium carbonate in distilled water and chloridric acid, the standard calcium solutions were obtained: 20 mg/l, 10 mg/l, 2.5 mg/l, and 1.25 mg/l.
For the spectrophotometer readings, 6 ml of the standard solutions or water from the samples were added by 2 ml of lanthanum chloride solution. Also, 6 ml of ultrapure water and 2 ml of lanthanum chloride was associated to obtain a reference solution without calcium.
After obtaining the standard, reference and test solutions, the reading was performed by the atomic absorption spectrophotometer. The calculi of the releasing were executed through the equation of the standard curve line. The reading of calcium releasing was executed at the same periods of the pH reading. Data were submitted to one-way analysis of variance and Tukey's test, both with level of significance at 5%.
Results
pH analysis
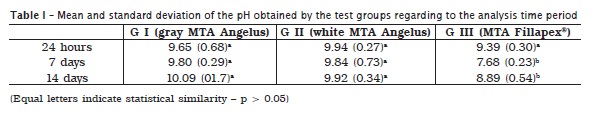
Table I shows the hydrogen ion releasing (pH) provided by the tested groups at the analysis periods of 24 hours, 7 and 14 days of immersion. At 24 hours, the test groups were similar among each other (p > 0.05). At seven and 14 days, Group III (MTA Fillapex) showed a pH significantly lower than gray and white MTA (p > 0.05). Gray and white MTA did not show any difference between each other (p > 0.05).
Calcium releasing analysis
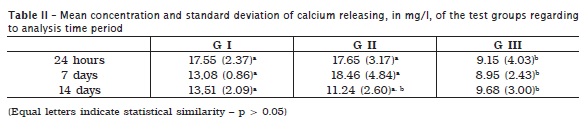
Table II shows the mean of calcium releasing, in mg/l, observed in the test groups regarding to the analysis at 24 hours, 7 and 14 days of immersion. At 24 hours and 7 days, Group III (MTA Fillapex®) demonstrated a significant lower calcium releasing than Groups I (gray MTA) and II (white MTA) (p < 0.05). At 14 days, Group III presented lower calcium releasing in comparison with Group I (p < 0.05).
Discussion
The use of materials that provide high alkalinity favors hard tissue mineralization as well as offers good antimicrobial activity 9. Accordingly, calcium reacts with carbonic gas, originating calcium carbonate, which will serve as a nucleus for initiating the calcification process 23. Therefore, by analyzing the alkalinization ability and calcium releasing, one indirectly evaluates the induction potential of mineralization provided by the material. Accordingly, MTA-based sealers present alkaline pH, high calcium ion releasing 25 and adequate biocompatibility 10.
The assessment of pH by pH meter 18 and calcium releasing by atomic absorption spectrophotometry 8, through the method of immersion of properly standardized polyethylene tubes in distilled water, is well established 16,25. Both the selection of polyethylene tube dimensions and the test time periods had as parameters the biocompatibility studies that have already evaluated similar materials 10. The placement of the materials inside root canals results in inaccurate results due to the difficult of standardization of the foramen opening and also because of the interference of root dentine itself 19. Once the reading was performed, the tubes were again immersed in another flask containing distilled water, avoiding therefore the ionic saturation that would interfere in the final outcomes 8,22.
At 24 hours, pH level increased both for gray and white MTA probably because just after the materials' handling they were immersed in water, circumstance in which the setting process is still occurring. This results in alkaline substances such as calcium hydroxide 11. These outcomes are in agreement with studies that used similar methodologies 8,25,27. At the other periods, pH was alkaline, without statistically significant differences between the two formulations.
The chemical composition of MTA Fillapex® is similar to that of Sealapex® sealer, even concerning to pH values 8. After the 24-hour period, MTA and MTA Fillapex® values were different, maybe due to MTA Fillapex® setting time, which has resin in its composition consequently reducing the medium alkalinization. This is also observed regarding to light-cured MTA cement 27 and resin endodontic sealers, e.g. Sealer26® 9.
The gradual reduction of calcium releasing by MTA is a tendency also found in other researches 3,8,27, although, this is still superior to that provided by MTA Fillapex®. At the 14th day of assessment, MTA Fillapex®'s calcium releasing was similar to white MTA, but lower than gray MTA. It is important highlighting that the gray formulation presents a tendency towards higher calcium releasing than the white one, at longer observation time-periods 3, possibly because the highest oxides concentration 11. At the initial periods, the highest concentration of calcium released may be explained by the chemical reaction itself between MTA and distilled water, originating calcium hydroxide as the final product 4,11.
MTA Fillapex® showed a tendency towards maintaining the calcium releasing relatively constant until 14 days. It has been verified that Sealapex® has the ability of presenting high calcium releasing after 25 to 30 days of mixing 8,24, which makes important to perform further studies on longer observation time periods.
Considering the methodology employed and literature discussed here, MTA Fillapex® should be only used as endodontic sealer, mainly in endodontic accidents of difficult access, since its physicochemical characteristics differ from gray and white MTA. Notwithstanding, the material presents an alkaline pH similar to that of the clinically and scientifically well-established sealers 8,24. Moreover, it has an adequate calcium releasing; however, further studies are necessary to certify its biocompatibility.
Conclusion
Based on the methodology employed in this present study, it can be concluded that:
• gray, white MTA and Fillapex® presents an alkaline pH and promote calcium releasing at all evaluated periods;
• MTA Fillapex®, after 24 hours, has a lower pH than the other materials' formulations (p < 0.05);
• gray and white MTA provided higher calcium releasing than Fillapex® (p < 0.05), except at 14 days, when white MTA's calcium releasing was similar to Fillapex® (p > 0.05).
Acknowledges
We thank to MSc. Larissa Tercilia Grizzo for the technical support; to PhD, MSc, DDS Marília Afonso Rabelo Buzalaf and Marco Antonio Hungaro Duarte from Bauru Dental School of the University of Sao Paulo for the permission of use of the devices.
References
1. Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005 Feb;31(2):101-3. [ Links ]
2. Bernabé PFE, Holland R, Morandi R, Souza V, Nery MJ, Otoboni Filho JA et al. Comparative study of MTA and other materials in retrofilling of pulpless dogs' teeth. Braz Dent J. 2005 May-Aug;16(2):149-55.
3. Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod. 2006 May;32(5):425-8.
4. Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Pitt Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005 Apr;21(4):297-303.
5. Camilleri J, Gandolfi MG, Siboni F, Prati C. Dynamic sealing ability of MTA root canal sealer. Int Endod J. 2011 Jan;44(1):9-20.
6. Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005 Sep;31(9):665-8.
7. Cintra LTA, Moraes IG, Estrada BPF, Gomes-Filho JE, Bramante CM, Garcia RB et al. Evaluation of the tissue response to MTA and MBPC: microscopic analysis of implants in alveolar bone of rats. J Endod. 2006 Jun;32(6):556-9.
8. Duarte MAH, Demarchi ACCO, Giaxa MH, Kuga MC, Fraga SC, Duarte LCD. Evaluation of pH and calcium release of three root canal sealers. J Endod. 2000 Jul;26(7):389-90.
9. Estrela C, Sydney GB, Bammann LL, Felipe Júnior O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995 Jul-Dec;6(2):85-90.
10. Gomes-Filho JE, Watanabe S, Bernabé PFE, Costa MTM. A mineral trioxide aggregate sealer stimulated mineralization. J Endod. 2009 Feb;35(2):256-60.
11. Han L, Okiji T, Okawa S. Morphological and chemical analysis of different precipitates on mineral trioxide aggregate immersed in different fluids. Dent Mat J. May;29(5):512-7.
12. Holland R, Souza V, Murata SS, Nery MJ, Bernabé PFE, Otoboni Filho JA et al. Healing process of dog dental pulp after pulpotomy and pulp covering with mineral trioxide aggregate or Portland cement. Braz Dent J. 2001 Jul-Dec;12(2):109-13.
13. Holland R, Mazuqueli L, Souza V, Murata SS, Dezan Júnior E, Suzuki P. Influence of the type of vehicle and limit of obturation on apical and periapical tissue response in dogs' teeth after root canal filling with mineral trioxide aggregate. J Endod. 2007 Jun;33(6):693-7.
14. Huffman BP, Mai S, Pinna L, Weller RN, Primus CM, Gutmann JL et al. Dislocation resistance of ProRoot Sealer, a calcium root canal sealer, from radicular dentine. Int Endod J. 2009 Jan;41(1):34-46.
15. Islam I, Chng HK, Yap AUJ. Comparison of the physical properties of MTA and Portland cement. J Endod. 2006 Mar;32(3):193-7.
16. Iunes AG, Iunes AG, Kuga MC, Campos EA, Tanomaru-Filho M, Kuga GK. Avaliação da capacidade de vedamento e pH do tampão apical com cimento Portland branco acrescido de radiopacificadores convencionais. RSBO. 2010 Jul-Sep;7(3):275-80.
17. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006 Jun;32(6):569-72.
18. Kuga MC, Pirolla MO, Freitas PC, Sant'Anna-Junior A, Guerreiro-Tanomaru J, Só MVR. Avaliação in vitro do pH do hidróxido de cálcio usado como medicação intracanal em associação com clorexidina e racealfatocoferol. RFO. 2010 May-Aug;15(2):150-4.
19. Kuga MC, Campos EA, Resende T, Camargo MF, Sant'Anna-Filho A, Monteiro JCC. Influência da dentina na liberação de íons hidrogênio em pasta de hidróxido de cálcio associada à clorexidina. Persp Oral Sc. 2010 Aug;2(2):27-30.
20. Lee SJ, Monsef M, Torabinejad M. Sealing ability of mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993 Nov;19(11):541-4.
21. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review. Part I: chemical, physical, and antibacterial properties. J Endod. 2010 Jan;36(1):16-27.
22. Santos AD, Moraes JCS, Araújo EB, Yukimitu K, Valério-Filho V. Physico-chemical properties of MTA and a novel experimental cement. Int Endod J. 2005 Jul;38(7):443-7.
23. Seux D, Couble ML, Hartmann DJ, Gauthier JP, Magloire H. Odontoblast-like cytodifferentiation of human dental pulp in vitro in presence of a calcium hydroxide-containing cement. Arch Oral Biol. 1991 Feb;36(2):117-28.
24. Silva LAB, Leonardo MR, Silva RS, Assed S, Guimarães LEL. Calcium hydroxide root canal sealers: evaluation of pH, calcium ion concentration and conductivity. Int Endod J. 1997 May;30(3):205-9.
25. Tanomaru-Filho M, Faleiros FBC, Saçaki JN, Duarte MAH, Guerreiro-Tanomaru JM. Evaluation of pH and calcium ion release of root-end filling materials containing calcium hydroxide or mineral trioxide aggregate. J Endod. 2009 Oct;35(10):1418-21.
26. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993 Dec;19(12):591-5.
27. Vivan RR, Zapata RO, Zeferino MA, Bramante CM, Bernardineli N, Garcia RB et al. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Sur Oral Med Oral Path Oral Radiol Endod. 2010 Aug;110(8):250-6.
28. Weller RN, Tay KCY, Garrett LV, Mai S, Primus CM, Gutmann JL et al. Microscopic appearance and apical seal of root canals filled with gutta-percha and ProRoot Endo Sealer after immersion in a phosphate-containing fluid. Int Endod J. 2008 Nov;41(11):977-86.
 Correspondence:
Correspondence:
Milton Carlos Kuga
Avenida Saul Silveira, n. 5-01.
CEP 17018-260 – Bauru – SP – Brasil
E-mail: kuga@foar.unesp.br
Received for publication: January 12, 2011
Accepted for publication: February 23, 2011













