Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.8 no.3 Joinville Jul./Set. 2011
ORIGINAL RESEARCH ARTICLE
Utilization of a biochemical kit for detection of C-reactive protein (CRP) in the saliva of periodontal disease individuals
Anaila BaroniI; Juliana Marchioro SouzaI; Maria Fernanda TorresI; Paulo Henrique TomazinhoI; João Armando BrancherI
I School of Dentistry, Positivo University – Curitiba – PR – Brazil
ABSTRACT
Introduction: Periodontal disease (PD) is a chronic inflammatory process that occurs in response to infection from bacteria in dental plaque. PD affects and destroys the periodontal tissues causing teeth loss. It is also associated to systemic diseases. C-reactive protein (CRP) is a protein produced by the liver and released into the blood during the acute phase of inflammation. Therefore, CRP is very used as a marker for inflammation process. Studies on the presence of CRP in the saliva of the subjects with PD do not exist. Objective: The aim of this study was to test a biochemical kit for CRP detection in blood plasma to monitor CRP in saliva of PD subjects. Material and methods: Saliva was collected from 40 individuals, both sexes, from 20-45 years-old, divided into two groups: Test Group – PD subjects (TG; n = 20) and Control Group (CG n = 20), without PD. The following salivary parameters were analysed: buffer capacity (BC), salivary flow (SF), pH, urea, total proteins, and CRP. Results: pH, SF and BC values were considered normal in both groups. The urea concentration was higher in TG (27.4 mg/dl ± 10.03) than CG (22.9 mg/dl ± 8.3). However, the concentration of total proteins was higher in CG (201.2 ± 100 mg/dl) than TG (155.0 ± 95 mg/dl). CRP was detected in 11 PD subjects and in eight subjects without PD. Conclusion: There were no significant differences between the two groups in relation to SF, pH and BC. However, in PD subjects' saliva, urea values increased and total proteins decreased. The biochemical kit detected CRP in subjects' saliva of both groups.
Keywords: periodontal disease; saliva; C-reactive protein.
Introduction
Periodontal disease constitutes an oral physical pathological disorder contributing to the evolution of systemic diseases 8. Several risk factors are pointed out as the causative agents, including specific bacteria, smoke, systemic diseases, genetic factors, among others; however, it seems that the main event initiating PD is the individual's chronic exposition to a pathogenic oral microflora existing in bacterial biofilm 6,29.
The chronic presence of the bacterial biofilm initiates a series of host's defense events which are part of the unspecific immunologic response intending to eliminate or neutralize the aggressive agent and essential for the damaged tissue 4, whose effects are not limited to the oral environment 3. PD's clinical manifestations comprise gingival inflammation – characterized by swelling, redness, and bleeding – formation of periodontal pockets, destruction of collagen fibers and periodontal ligament, as well as loss of bone support, which consequently leads to tooth loss 18,24.
Although PD's local effects are well established, the researchers increasingly searched to determine a connection among the events occurring in oral cavity and the systemic inflammatory processes, such as renal insufficiency 15, atherosclerosis 3, and diabetes 18.
C-reactive protein (CRP) is produced by the liver and released into the blood during the acute phase of the inflammatory process 12. In patients presenting systemic infections, plasmatic CRP levels may increase significantly 17. In vitro and in vivo studies evidenced CPR increase in inflamed tissues 11, atherosclerotic vases 17, and blood plasma of PD patients 27. Moreover, individuals who have high levels of plasma CRP demonstrated an increased risk of chronic diseases, including cardiovascular disorders 25.
CRP is very employed as an inflammatory process marker due to the easiness in determining the plasma concentration; however, there is a lack of studies on CRP increase in saliva of PD's patients.
Saliva is a fluid of glandular origin that covers the oral surfaces and has very varied physical chemical properties. Its secretion is induced by psychic, mechanical, physical, chemical, and biological stimulus 16. Its components include minerals, proteins, mucosal transudate, and gingival sulcus exsudate 7. Some of saliva's functions are cleanness, protection and oral pH maintenance 16. The use of saliva as a diagnostic method of several diseases significantly progressed in the last years. Through salivary examination, it is possible to detect the presence of oral microorganisms, body's chemical substances, and immunological markers, as well as the possibility of monitoring oral and systemic diseases 31. The main advantages of salivary fluid instead of blood use for diagnosing are its easy access and noninvasive collection.
It is known that PDs may cause qualitative and quantitative alterations in the salivary components; however, there is no report in literature on the presence of CRP in the saliva of PD patients. Therefore, the aim of this study was to test a biochemical kit, used in clinical analysis laboratory, for CRP detection in blood plasma to monitor CRP in saliva of PD subjects.
Material and methods
This study was approved by the Ethical Committee in Research of the Positivo University, under protocol number #41/2006. Forty subjects were evaluated, both sexes, from 20 to 45 years-old, divided into two groups: test group (TG) comprises 20 patients treated in the Clinics of Periodontology of the Positivo University diagnosed as chronic PD, according to the criteria of the American Academy of Periodontology 1; control group (CG) comprises 20 volunteers who were undergraduates of the School of Dentistry of the Positivo University, matched in sex and age with TG individuals. All participants underwent a clinical, radiographic examination and anamnesis. Exclusion criteria comprise any person who had been taking hyposalivation-inductor medicaments; had initiated PD treatment, presented a systemic disease or had not been able to undergo the total saliva collection by the established technique.
Samples of total saliva were collected by the spitting technique, according to the description of Navazesh 21. The patient was instructed to spit all the saliva produced in a sterile universal container, previously numbered and weighed. The saliva samples were stored into a Styrofoam container containing ice inside it and sent to a lab for biochemical analysis. Salivary pH was measured with aid of a potentiometer (Mettler Toledo 320, SP, Brazil). The determination of the salivary buffer capacity (SBC) was executed by titration, and the salivary flow volume (SFV) was determined by the method developing by Banderas-Tabaray 2.
Sialochemical assessment was performed by enzymatic colorimetric tests for urea (Katal Biotec. Ind. Com. Ltda., Belo Horizonte, MG, Brazil) and total proteins (PROTI 2, Wiener Lab., Argentina). Urea and total protein tests were executed three times for each saliva samples, following the manufacturer's instructions with regards to the biochemical preparation of the samples. The readings were carried out in a spectrophotometer (Siel 500). To detect the CRP presence in saliva, a PCR L/B (Laborclin Produtos para Laboratórios Ltda., Pinhais, PR, Brazil) was used. The saliva sample was placed into contact with the kit's reagent, which contains latex particles covered by anti-CRP antibody. If CRP is present, a visible agglutination of the latex particles will be seen by naked eye.
Results
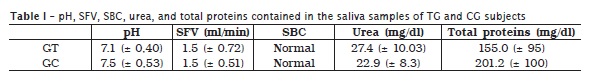
The saliva samples of 40 subjects were analysed, 20 PD patients (TG) and 20 healthy volunteers (CG, without PD). The obtained results are summarized in table I. It was verified that there are no statiscally significant differences in either pH or SFV values, between CG and TG. SBC was considered as normal for all the 40 study's participants. Salivary urea concentration was higher in TG than CG.
Salivary urea mean values and standard deviations were 27.4 mg/dl (± 10.03) and 22.9 mg/dl (± 8.3) for TG and CG, respectively. Contrastively, CG salivary total proteins (201.2 ± 100 mg/dl) were higher than TG (155.0 ± 95 mg/dl).
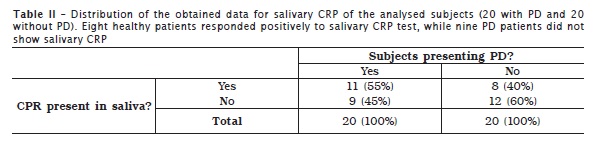
CPR test was negative, that is, the tested protein was not detected in 12 CG subjects; however, CRP was present in the saliva of eight CG subjects. In PD subjects (TG), 11 showed salivary CRP while 9 did not present it (table II).
Discussion
Studies on PD pathogenesis should evaluate the biochemical and immunological factors influencing on the disease's severity, progress, and prognosis. Several body fluids can be used to diagnose PD because it is possible to find inflammatory markers within them. Among these fluids are the saliva, gingival sulcus fluid, and blood plasma 5,14.
The saliva presents some advantages, with an easy and noninvasive collection, and can replace the blood as a diagnose resource 10. Saliva's biochemical analysis as well as hematologic examinations has two main goals: to identify the presence of pathology and to evaluate the disease's diagnosis during treatment 28.
According to Lagerlof and Oliveby 16, the saliva contains physicochemical specific properties, in addition to blood components and excretion products, such as: medicaments, drugs, and microbial activity products. One of the most important functions of the saliva is the SBC, i.e., the property of maintaining oral pH between 6.0 and 8.5. Such function is attributed to the action of salivary proteins and ions 28. In this study, we observed that all subjects presented a normal SBC, and the salivary pH varied within the limits already assessed by literature.
Saliva's mechanical action, promoting oral cavity washing, is also of great relevance to oral health. Saliva's efficiency in this aforementioned promotion is measured by SFV, i.e., the amount of saliva produced by the person. SFV of this study's participants was considered as normal and, there were no statistical differences between groups.
Urea, the residue of amino acid catabolism, is excreted mostly in urine; however, it can also be eliminated by saliva, at a rate of 20 mg/dl 20. In saliva of final-stage renal patients, salivary urea levels are high due to the lack of elimination by the kidneys as well as the oral protein hydrolysis by specific bacteria, which may be responsible for PD progression. This would explain the highest dental calculus formation in chronic renal patients 30.
In this study, an increase in salivary urea rate and decrease in the amount of total proteins of PD patients was observed. This finding suggests that salivary proteins or glycoproteins are being degraded by oral bacteria and used as nitrogen resource.
Several studies were performed attempting to show whether saliva could be employed as a diagnostic tool. Kalk et al. 13 demonstrated that Sjögren's syndrome patients presented salivary alterations. Gandara et al. 9 found sialochemical differences in stimulated total saliva of patients presenting oral liken planus. On the other hand, Oba et al. 23 compared saliva and serum samples by immunoassay tests aiming to detect anti-HVA IgM, IgA and total antibodies and found a high agreement between the results of blood and saliva samples. In other study, paired blood and oral-fluid samples were obtained from 853 individuals to assess the suitability of using oral-fluids in the prevalence determination of immunity to vaccines. The authors suggested that the saliva can replace serum as a diagnostic resource 22.
The use of saliva as PD's diagnostic tool has been studied and several salivary markers have been recommended, including salivary proteins, phenotypic markers, cortisol, and even bacteria and its metabolism products 14. Due to its easy and fast determination, blood CRP has been an inflammatory marker of great interest 26 that contributes to evaluate the disease's progression. Slade et al. 27 and Santos et al. proved that blood CRP increases due to acute or chronic, oral or systemic inflammations; however, there is a lack of studies on the presence of CRP in PD patient's saliva.
This study tested a biochemical kit for CRP detection in blood plasma employed at clinical analysis laboratory. Because there are no reports in literature regarding to the use of this kit for CRP detection in saliva of PD patients, the aim of this study was to analyze whether the material would be efficient in revealing the presence of the protein in PD patients' saliva. We found positive results both for CG and TG patients. Eight subjects without PD presented CRP in saliva. This result interpretation demands a careful correlation between patient's clinical history and current clinical state, since a simple flu can increase the blood CRP levels and more than one assessment may be necessary for a correct evaluation of patient's relative risk 19,26.
The tested CRP kit was found to be efficient for assessing the presence of salivary CRP of the tested subjects. Notwithstanding, such protein was found in the saliva of patients with and without PD. Further studies are necessary to elucidate the causal mechanisms responsible for the CRP appearance in the saliva of patients without PD as well as its absence in the saliva of PD patients, seeking for statistically significant differences that would or would not justify this protein presence in apparently healthy patients.
Conclusion
Significant alterations in SFV, pH, and SBC were not observed between control and test groups. Salivary urea rate increased and total proteins decreased in patients without PD. The tested CRP kit revealed the presence of the salivary protein in both groups.
References
1. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999 Dec;4(1):1-6. [ Links ]
2. Banderas-Tarabay JA. Flujo y concentración de proteínas en saliva total humana. Salud Publica Mex. 1997 Sep;39(5):433-41.
3. Beck JD, Slade G, Offenbacher S. Oral disease, cardiovascular disease and systemic inflammation. Periodontol. 2000 Jun;(23):110-20.
4. Chen LS, Balakrishnan K, Gandhi V. Inflammation and survival pathways: chronic lymphocytic leukemia as a model system. Biochem Pharmacol. 2010 Dec;80(12):1936-45.
5. Chomyszyn-Gajewska M. Evaluation of chosen salivary periodontal disease markers. Przegl Lek. 2010 Oct;67(3):213-6.
6. Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000. 1997 Jun;(14):12-32.
7. Epstein JB, Scully C. The role of saliva in oral health and the causes and effects of xerostomia. J Can Dental Assoc. 1992 Mar;58(3):217-21.
8. Fowler EB. Periodontal disease and its association with systemic disease. Military Med. 2001 Jan;166(1):85-9.
9. Gandara BK, Izutsu KT, Truelove EL, Mandel ID, Sommers EE, Ensign WY. Sialochemistry of whole, parotid, and labial minor gland saliva in patients with oral lichen planus. J Dent Res. 1987 Nov;66(11):1619-22.
10. Gonzalez LFA, Sanches MCR. La saliva: revision sobre composicion, funcion y usos diagnósticos. Primera parte. Univ Odontol. 2003 Sep;(23):18-24.
11. Hatanaka K, Li XA, Masuda K, Yutani C, Yamamoto A. Immunohistochemical localization of C-rective protein-binding sites in human atherosclerotic aortic lesions by a modified strepdavidin-biotin-staining method. Pathol Int. 1995 Sep;45:635-41.
12. Hediund P. Clinical and experimental studies protein (acute phase protein). Acta Med Scand. 1961 Apr;361(1):123-9.
13. Kalk WW, Vissink A, Spijkervet FK, Bootsma H, Kallenberg CG, Nieuw Amerongen AV. Sialometry and sialochemistry: diagnostic tools for Sjögren's syndrome. Ann Rheum Dis. 2001 Dec;60(12):1110-6.
14. Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis: a review. J Clin Periodontol. 2000 Jul;27:453-65.
15. Khocht A. Periodontitis associated with chronic renal failure: a case report. J Periodontol. 1996 Nov;67(11):1206-9.
16. Lagerlof F, Oliveby A. Caries-protective factors in saliva. Adv Dent Res. 1994 Jul;8(2):229-38.
17. Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Review Circulation. 1999 Jul;100(1):96-102.
18. Lalla E, Cheng B, Lal S, Tucker S. Periodontal changes in children and adolescents with diabetes. Diabetes Care. 2006 Feb;29(2):295-9.
19. Machado ACP, Vadenal R, Cortelli JR. Doença periodontal e doença cardíaca: uma revisão dos mecanismos. Rev Bioc. 2004 Sep;10(3):153-9.
20. Mattioli TM, Koubik AC, Ribas MO, França BH, Brancher JA, Lima AA. Salivary flow rate, calcium, urea, total protein, and amylase levels in fanconi anemia. J Pediatr Hematol Oncol. 2010 Mar;32(2):46-9.
21. Navazesh M. Comparison of whole saliva flow rates and mucin concentration in healthy Caucasian young and aged adults. J Dental Res. 1992 Jul;71(6):1275-8.
22. Nokes DJ, Enquselassie F, Nigatu W, Vyse AJ, Cohen BJ, Brown DW et al. Has oral fluid the potential to replace serum for the evaluation of population immunity levels? A study of measles, rubella and hepatitis B in rural Ethiopia. Bull World Health Organ. 2001 Jan;79(7):588-95.
23. Oba IT, Spina AM, Saraceni CP, Lemos MF, Senhoras R, Moreira RC et al. Detection of hepatitis A antibodies by ELISA using saliva as clinical samples. Rev Inst Med Trop São Paulo. 2000 Jul;42(4):197-200.
24. Proctor R, Kumar N, Stein A, Moles D, Porter S. Oral and dental aspects of chronic renal failure. J Dental Res. 2005 Mar;84(3):199-208.
25. Ridker PM, Baker MT, Hennekens CH, Stampfer MJ, Vaughan DE. Alu-repeat polymorphism in the gene coding for tissue-type plasminogen activator (t-PA) and risks of myocardial infarction among middle-aged men. Arterioscler Thromb Vasc Biol. 1997 Sep;17(9):1687-90.
26. Santos WB, Mesquita ET, Vieira RMR, Olej B, Coutinho M, Avezum A. Proteína C-reativa e doença cardiovascular: as bases da evidência científica. Arq Bras Cardio. 2003 Apr;80(4):452-6.
27. Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000 Jan;79(1):49-57.
28. Slavkin HC. Toward molecularly based diagnostics for the oral cavity. J Am Dent Assoc. 1998 Aug;129(8):1138-43.
29. Socransky SS, Haffajee AD, Cugini MA. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998 Feb;(25):134-44.
30. Souza CRD, Libério AS, Guerra RM, Monteiro S, Silveira ED, Pereira AL. Avaliação da condição periodontal de pacientes renais em hemodiálise. Rev Assoc Med Bras. 2005 Oct;51(5):285-9.
31. Streckfucks CF, Bigler LR. Salivary glands and saliva: saliva as a diagnostic fluid. Oral Diseases. 2002 Aug;8(3):69-76.
 Correspondence:
Correspondence:
João Armando Brancher
Rua Venezuela, n. 54 – Bacacheri
CEP 82510-100 – Curitiba – PR – Brasil
E-mail: brancher@up.edu.br
Received for publication: January 31, 2011
Accepted for publication: February 25, 2011













