Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.8 no.3 Joinville Jul./Set. 2011
ORIGINAL RESEARCH ARTICLE
Analysis of fluoride concentration in solutions prepared at dispensing pharmacies
Eduardo PizzattoI; Estela Maris LossoI; Melina Carolina MirandaI; Vivian Danielle de SouzaI; Felipe Belmonte ArchettiI
I School of Dentistry of Positivo University – Curitiba – PR – Brazil
ABSTRACT
Introduction: Fluoride plays an important role in oral health promotion and is considered important in dental caries prevention both in children and adults. Fluoride is widely used at high-risk conditions of caries, when the use of fluoride-containing mouthwashes is recommended, considering that fluoride itself reduces the risk of dental caries. Objective: To evaluate the fluoride concentration in solutions prepared at different dispensing pharmacies in the city of Curitiba – PR, Brazil. Material and methods: The analysis of fluoride concentration was preformed through Ion Chromatography method (DIONEX). Results: The results obtained through this analysis showed that all solutions presented fluoride concentration above that required in the dentist's prescription, varying between 5.48% and 24.02% more fluoride, at absolute concentration. Conclusion: This finding highlights the increasing risk of fluoride acute intoxication in cases of accidental ingestion of the solution.
Keywords: topical fluorides; dental caries; fluoride poisoning.
Introduction
Scientific evidences have been increasingly demonstrating that fluoride exerts its anticaries effect on the interface biofilm/saliva/tooth, during the periods of enamel dissolution. This also means that fluoride concentration itself, at either topical solutions or hard dental tissues, is not a highly significant determinant of the predictable anticaries effect 17. Since fluoride anticaries effect is also related to its presence within oral fluids, even at low concentrations, its concentration kinetics in saliva plays an important role in the dynamic of the caries controlling process 1.
Because of the highest fluoride concentrations of topical methods, applied locally at concentrations starting from 100 parts per million (ppm), the fluoride incorporation to enamel's surface occurs, mainly as calcium fluoride. When calcium fluoride is formed on tooth surface, it is covered by calcium, phosphate and salivary proteins, constituting a type of protective cover which retards the pattern of the compound dissolution, resulting in slowly fluoride releasing 5.
Mouthwashes were developed in the 1950s, attempting to discover efficient, simple, and fast procedures for fluoride applications 17. The first clinical assessments of fluoride mouthwashes were carried out in Scandinavia, based on 0.2% sodium fluoride solutions, at every two weeks 12,17. Neutral sodium fluoride is the most preferred agent due to its effect, easy preparation and storage, low cost, pleasant flavor, as well as lack of ability to stain 12.
The fluoride agent applications on tooth surface presents calcium fluoride (CaF2) as the main product, stored as a fluoride ions' reservoir available to be released in cariogenic challenges at oral environment. Concerning to dental caries prevention, it seems very interesting to use substances capable of remaining for longer time periods in contact with tooth enamel, depositing soluble CaF2 on it, at greater amounts 11. Therefore, the presence of topical fluoride is, undoubtedly, the most important via of this element use in dental caries prevention 17. Notwithstanding, there are several ways of topical fluoride application, comprising either professional or over-the-counter solutions 1.
At first, mouthwash should be accomplished every day at bedtime to potentialize fluoride pharmacokinetics aspects 2. Mouthwash solutions with pleasant taste must be avoided, because they increase both the risk of ingestion and the velocity of fluoride elimination due to salivary flow stimulation 4.
Fluoride mouthwashes must obey the regulations specified by the Brazilian Health Surveillance Agency (Regulation no. 22 from December 22, 1989), which specifies the quality and amount requisites for such products 3.
These products for daily use, containing either sodium fluoride or sodium monofluorophosphate, only will be registered at the National Division of the Brazilian Health Surveillance Agency if the manufacturer presents the following documents:
• the concentration of soluble fluoride, ionic or ionizable in the product ranges from 202.5 ppm (minimum) and 247.5 ppm (maximum);
• the fluoride compound within the product must react with tooth's enamel and/or dentin;
• the product labels must show the fluoride compound chemical formula within the mouthwash, its concentration (expressed in ppm) and respective indications; how to use; manufacture and expiration date.
The fluoride mouthwashes are contraindicated for children in preschool age because they are not capable of spitting. In addition to this fact, 10 to 20% of the product is normally ingested, which increases the risks of dental fluorosis, mainly when associated to fluoride water 2,12. According to Tenuta and Cury (2008) 6, fluoride mouthwashes should be recommended only for children above 6 years-old. However, even these children must be supervised and instructed to expectorate, because a small amount of the product is almost always ingested 18.
After the use of a topical fluoride solution, a great amount of fluoride is leached by saliva and then swollen; however, small amounts of fluoride may be stored and later released 8. Therefore, fluoride releasing by saliva is subjected to the amount of calcium fluoride formed on either biofilm or tooth surface, as well as to its degree of dissolution by saliva 6.
Currently, fluoride mouthwash indication is based much more on the individual's caries risk or activity. Notwithstanding, fluoride mouthwash may be important collectively, depending on the analysis of each situation 1. A fluoride mouthwash program should be recommended 10 for patients:
• who failed in attempting to reach an acceptable level of oral hygiene;
• who present a caries risk localized in areas showing biofilm accumulation (orthodontic brackets, removable prosthesis, and restoration margins);
• who present gingival retraction and susceptibility to root caries;
• who present reduced salivation (due to surgery, medication, radiotherapy);
• who present rampant caries lesions and increase of caries activity.
Nevertheless, fluoride mouthwash method ended up restricted to well-defined groups at high risk of caries disease, particularly in populations presenting high caries activity 17. Therefore, fluoride mouthwashes are now used frequently as part of an individual oral health program 17.
Although over-the-counter fluoride mouthwashes exist, fluoride solutions may be prescribed by the dentist to be prepared at dispensing pharmacies. Generally, over-the-counter fluoride mouthwashes contain flavorings and preservatives, and many of them associate fluoride with antibacterial substances capable of showing clinical implications 1.
In this context, the aim of this study was to evaluate the fluoride concentration within 0.2% sodium fluoride solutions prepared at different dispensing pharmacies of the city of Curitiba, Parana, Brazil.
Material and methods
The sample was composed of fluoride solutions (0.2% sodium fluoride) prepared at 10 different dispensing pharmacies of the city of Curitiba (Parana, Brazil), which were numerated not to be identified. Each bottle contained 1.000 ml of the aforementioned solution. These solutions were separated at the laboratory, pipetted and inserted into 5 ml bottles. This study was carried out at the Analytics Central Laboratory of Positivo University.
The analysis were performed through an ion chromatograph (model ICS-90, Dionex), with AS40 automated sample equipped with isocratic bomb. The volume of sample injection was 1 ml/min, and the analytics columns include one Dionex AG9-HC pre-column and one Dionex AS9-HC pre-column. Eluent conductivity was suppressed by the anion suppressor Dionex AMMS III (4 mm).
To data interpretation, Chromeleon 6 software, connected to the device, was employed.
All aqueous solutions were prepared with deionized, ultrapure water obtained through milli-Q system. Sodium carbonate was the eluent (0.5 molar) and 75 ml of sulphuric acid (20 x) was the regenerant reagent.
Results
After the analysis, it was observed that all evaluated solutions presented fluoride amount above that required in the prescription, as seen in table I.
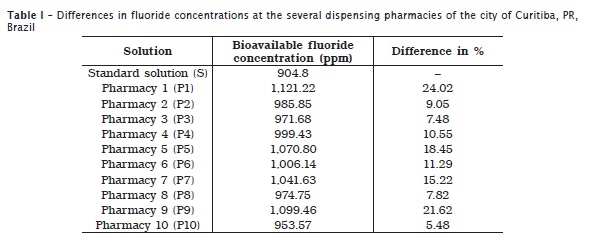
Based on the obtained results, all the solutions surpassed 0.2% of the required concentration. This is capable of causing a mild to severe acute intoxication if a patient swallows a considerable dose, therefore, having serious consequences.
The intoxication gravity will depend on the amount swollen vs. the fluoride concentration within the solution. For each case of intoxication, the dentist will act according to patient's symptoms. Solutions P1, P9, P5, in this order, exhibited the highest differences in comparison with the standard solution. Consequently, they offer the highest risk for a person who accidently ingested them, may causing acute intoxication.
Discussion
Daily fluoride mouthwashes has proved efficacy in caries disease prevention. However, the subject's individual fluoride consumption should be considered because in addition to mouthwashes, the patient is generally using fluoride dentifrices twice to three times a day and consuming fluoride water. Therefore, fluoride mouthwashes – as an additional method for caries prevention – should be only indicated for those patients who are more likely affected by this disease. People presenting reduced salivary flow, using orthodontic devices that make biofilm removal difficulty or having high ingestion frequency of fermentable carbohydrates are prone to use fluoride mouthwashes as adjuvants for caries prevention 16.
Although fluoride has been considered an efficient cariostatic compound, it presents limitations regarding to both the individual and collective use. Individually, fluoride success in caries prevention depends on the establishment of a new habit by the patient; it is known that only 50% of the patients will continue its use. Consequently, it is important to prescribe daily mouthwash. Generally, it comprises 0.05% NaF through dispensing formulas and commercial products 2.
Collectively, fluoride mouthwash programs require a longer time, consequently, a higher patient's compliance. Because the fluoride dosage is relatively high (approximately 2.5 mg of F for 0.05% daily mouthwash and 10 mg of F- for 0.2% NaF weekly mouthwash), mouthwashes must always be executed with adult supervision. Daily mouthwashes seem to have a higher patient's compliance 4. Considering daily fluoride mouthwash programs, they demonstrated to have better results than weekly mouthwash programs. Also, it does not seem to be a difference between the efficacy of fluoride acidulated and neutral solutions 17.
Today, caries risk is no longer the same as in 1950s, when fluoride mouthwashes were instituted, consequently, it is necessary an individual assessment of caries risk, by diagnostic tools aiming to identify the risk's main causes. 0.05% NaF daily mouthwashes are suggested for patients at high risk, in combination with other preventive measurements, such as dental biofilm controlling and dietary sugar restriction 9.
Fluoride mouthwash maintains a higher fluoride amount in saliva than fluoride dentifrice depending on the mode of fluoride use in each method. Generally 0.7g of dentifrice is placed on toothbrush, resulting in 1.0 mg of fluoride amount per toothbrushing. In 15 ml of mouthwash, the amount of available fluoride is 3.4 mg. Accordingly, fluoride mouthwashes are more effective methods than dentifrices regarding to fluoride retention in saliva 14.
Delbem et al. (2003) 7 analysed 14 fluoride solutions, among which 50% exhibited statistically significant differences, with fluoride concentrations above those printed in the bottle's label. Considering the Brazilian Health Surveillance Agency regulations, only three products showed values above those determined, with one presenting almost twice the value of the recommended fluoride amount.
Rodrigues et al. (2002) 13 evaluated the fluoride concentration of six over-the-counter trademarks found in supermarkets and verified that none of them met the Brazilian Health Surveillance Agency regulations concerning to the expected concentration. Tabchoury et al. (2005) 15 examined fluoride solutions produce at dispensing pharmacies and found that most of them presented a fluoride concentration close to the expected value, except for the product prepared in one pharmacy at a concentration five times smaller than expected.
Conclusion
Based on the literature review as well as on data obtained in the experimental phase, it can be concluded that:
• All solutions presented fluoride concentration above to that prescribed, which increases the risk of acute intoxication in case of accidental ingestion of these solutions;
• Further studies should be performed using this same methodology to verify whether such alterations would or would not be isolated facts.
References
1. Baratieri LN. Odontologia restauradora: fundamentos e possibilidades. São Paulo: Santos; 2001. 740 p. [ Links ]
2. Bottino MA, Feller CF. Atualização na clínica odontológica: o dia a dia do clínico geral. São Paulo: Artes Médicas; 1992. 449 p.
3. Brasil. Ministério da Saúde. Portaria n.º 22, de 20 de dezembro de 1989. Brasília; 1989.
4. Buischi YPB. Promoção de saúde bucal na clínica odontológica. São Paulo: Artes Médicas; 2000. 359 p.
5. Corrêa MSNP. Odontopediatria na primeira infância. São Paulo: Santos; 1998. 679 p.
6. Dawes C, Weatherell JA. Kinetics of fluoride in the oral fluids. J Dent Res. 1990;69:638-44.
7. Delbem ACB, Sassaki KT, Castro AM, Pinto LMCP, Bergamaschi M. Assessment of the fluoride concentration and pH in different mouthrinses in the Brazilian market. J Appl Oral Sci. 2003;11(4):319-23
8. Duckworth RM, Morgan SN. Oral fluoride retention after use of fluoride dentifrices. Caries Res. 1991;25(2):123-9.
9. FDI Commission. Mouthrinses and dental caries. Int Dent J. 2002;52(5):337-45.
10. Harris NO, Christen AG. Primary preventive dentistry. 4. ed. Stamford: Appleton & Lange; 1995. 635 p.
11. Medeiros UV, Mendonça LVD. Formação in situ de fluoreto de cálcio a partir da utilização de vernizes fluoretados. RBO. 1997;54(2):102-6.
12. Murray JJ. O uso correto de fluoretos na saúde pública. São Paulo: Santos; 1992. 131 p.
13. Rodrigues LKA, Dalcico R, Gomes VE, Zanin ICJ, Nascimento MM, Duarte S. Análise de flúor em enxaguatórios bucais encontrados no comércio brasileiro e o uso de eletrodo íon específico. Rev Pós-Grad. 2002;9:142-8.
14. Serra MC, Cury JA. Cinética do flúor na saliva após o uso de dentifrício e bochechos fluoretados. APCD. 1992;46(5):875-8.
15. Tabchoury CPM, Pierobon CN, Cury JA. Concentration and bioavailability of fluoride in mouthrinses prepared in dispensing pharmacies. J Appl Oral Sci. 2005;13(1):41-6.
16. Tenuta LMA, Cury JA. Evidências para o uso de fluoretos em Odontologia: parte II – meios de usar fluoretos em Odontologia. Jornal da ABO. 2008;116:14-5.
17. Thylstrup A, Fejerskov O. Cariologia clínica. São Paulo: Santos; 2005. 421 p.
18. Whitford GM. The physiological and toxicological characteristics of fluoride. J Dent Res. 1990;69:539-57.
 Correspondence:
Correspondence:
Eduardo Pizzatto
Mestrado em Odontologia – Universidade Positivo
Rua Professor Pedro Viriato Parigot de Souza, n. 5.300 – Campo Comprido
CEP 81280-330 – Curitiba – PR – Brasil
E-mail: epizzatto@up.com.br
Received for publication: December 16, 2010
Accepted for publication: February 12, 2011













