Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.8 no.4 Joinville Out./Dez. 2011
ORIGINAL RESEARCH ARTICLE
Clinical evaluation of 4% hydrogen peroxide bleaching in mandibular teeth
Lais Dalmagro PeruchiI; Neimar SartoriI; Guilherme Carpena LopesI; Andressa BallarinI; Camila AmbrosiI; Jussara Karina BernardonI
I Department of Dentistry, Federal University of Santa Catarina – Florianopolis – SC – Brazil
ABSTRACT
Introduction: Bleaching agents have been constantly introduced into market; however, the efficacy of new hydrogen peroxides still needs to be evaluated. Objective: The aim of this study was to evaluate in vivo the effectiveness of 4% hydrogen peroxide bleaching agent in color change, bleaching maintenance, tooth sensitivity, and patients' satisfaction. Material and methods: Forty subjects were selected, lower bleaching trays were constructed, and the patients were instructed to apply 4% hydrogen peroxide bleaching agent at home. Shade measurements of the mandibular anterior teeth were carried out with a spectrophotometer and Vita Classical shade guide (VITA) at the following moments: baseline, 14 days, after patients' satisfaction, and after 90 days. In addition, tooth sensitivity was evaluated using a visual analogue scale (VAS) at the first 14 days. Shade measurements were submitted to repeated measures ANOVA with Bonferroni adjustment and level of significance set at 5%. Results: Statistically significant differences were found for mandibular tooth shade after at-home bleaching (p < 0.001). After 14 days of bleaching, 90% of the subjects were not pleased with the achieved bleaching; however, after 27 days most of the patients reported to be satisfied. Tooth sensitivity was reported to range from 1.01 in a scale from 0-10. Conclusion: At-home bleaching using 4% hydrogen peroxide is effective in lower teeth with reduced tooth sensitivity.
Keywords: tooth bleaching; hydrogen peroxide; patient satisfaction.
Introduction
Tooth bleaching becomes a very popular due to be an effective, safe, low-cost, conservative treatment to obtain vital tooth whitening 16,24. Tooth whitening mechanism is not totally understood 21; however, it is believed that the bleaching agent (hydrogen peroxide) is decomposed to produce oxygen (O+) and hydroxyl radicals (HO2-) 20. These free radicals attack tooth dark pigment molecules (organic macromolecules formed by aromatic rings), to obtain stability by breaking them into smaller, less complex and clearer molecules than the original ones 13.
Among bleaching techniques for vital teeth, at-home and in-office whitening are emphasized 14. At-home bleaching main advantages are effective outcomes, technique simplicity, low cost, and to be executed out of dental office 27,29; the disadvantages are color regression 6, long-time treatment, which contributes to tooth sensitivity during treatment 27.
At-home bleaching treatment comprises the application of a bleaching agent gel, based on carbamide peroxide at 10% to 22% concentration or hydrogen peroxide ranging from 4% to 9% concentration placed onto a customized tray, which is daily used by the patient. The original technique advocates the bleaching agent application for six to eight-hour daily regimen, during the night 11. Notwithstanding, Matis et al. 19 demonstrated that after two hours of bleaching, only 50% of the active agent is available; after 10 hours, bleaching agent availability decreases to 10%. Consequently, depending on bleaching agent concentration, some manufacturers recommended only 30 minutes of daily use of bleaching agent.
Scientific evidences showed that tooth bleaching can reduce tooth's microhardness, fracture toughness and calcium and phosphate concentrations 1, as well as increase enamel's superficial roughness 18,23. Some compounds may be added to bleaching gels, e.g. potassium nitrate and sodium fluoride, to act as desensitizing agents; sodium fluoride also allows tooth remineralization 10. Currently, some manufacturers start to add calcium to bleaching agent gels, aiming to decrease tooth enamel's demineralization process.
Therefore, the aim of this clinical study was to assess the effects of a 4% hydrogen peroxide bleaching agent containing potassium nitrate, sodium and calcium fluoride on color change, whitening maintenance, tooth sensitivity, and patient's satisfaction.
Material and methods
This research was approved by Human Research Ethics Committee. Forty volunteers were selected according to the inclusion and exclusion criteria listed in table I. All selected volunteers received detailed information on the research and the technique's goals and risks. Following, the participants signed a Informed Consent, meeting the Resolution number #196, from October 10, 1996, by the Brazilian Council of Health/Ministry of Health, Brasilia, DF, Brazil.
At first appointment, a professional dental prophylaxis was executed, followed by filling in the specific tooth bleaching file and upper and lower arch impressions with alginate (Avagel, São Paulo, Brazil). The impressions were poured-up with die stone (Vigodent S.A. Indústria e Comércio Bom Sucesso, Rio de Janeiro). After the stone setting, working casts were adequately cut. Lower and upper trays were made and cut 2 mm above the gingival margin.
Prior to bleaching onset, color measurements of tooth #33, #32, #42, and #43 were executed by using a shade guide (Vita Classical, Vita Zahnfabrik, Bad Säckingen, Germany) and spectrophotometer (Vita Easyshade, Vident, Brea, USA) and recorded on a specific file. A demonstration of how the bleaching agent should be placed into the tray was performed patients were instructed to use the bleaching gel for two hours per day, according to the manufacturer's instructions. At that moment, patients received one syringe of 4% hydrogen peroxide bleaching agent gel (White Class, FGM, Joinville, Brazil) and the customized tray for lower arch whitening. Also a visual analogue scale (VAS) was given to the patient to register tooth sensitivity during the first 14 days of treatment. Patients were instructed to classify tooth sensitivity in a scale from 0 to 10, varying from "no discomfort at all" to "extremely unpleasant" 25.
The first follow-up appointment was performed 14 days after the treatment onset. At this evaluation, the shade measurements by using the shade guide and spectrophotometer were executed, following the same parameters of the baseline assessment. After shade measuring, the participants answered a questionnaire aiming to reveal their satisfaction degree with tooth whitening at 14 days of treatment 7. If patient were satisfied with 14-day treatment and would not want to continue it, the bleaching agent syringe was returned. On the other hand, if patient were not satisfied, we oriented to continue treatment and a new appointment was scheduled.
For patients who wanted to continue treatment, a new follow-up appointment was performed after patient satisfaction with tooth whitening outcome. This assessment followed the same pattern of the previous ones. Twelve weeks after bleaching treatment onset, a new assessment was scheduled to verify tooth whitening maintenance over time. At this appointment, patient received the customized upper tray to execution of upper arch bleaching.
Visual shade measurement was standardized through the controlling of light environment. For this purpose, we used a light temperature of 6,500 K which corresponding to day light, at a distance of 25 cm from patient's face. Additionally, all participants wore a neutral gray apron over their clothes to avoid interference during the assessment. The shade measurement was always carried out at tooth's medium third. To assure an accurate measurement, the shade guide was ordered by value, as follows: B1, A1, B2, D2, A2, C1, C2, D4, A3, D3, B3, A3.5, B4, C3, A4 and C4 (in which B1 corresponded to color 1 and C4 to color 16). Shade value was covered by an adhesive tape to decrease examiner's error.
Objective measurement of shade was performed with a spectrophotometer following the manufacturer's instructions, with the device's tip placed onto labial surface of the medium third of the evaluated lower teeth. To standardize the tooth area which would be evaluated with the spectrophotometer, a device made out of orthodontic wire was constructed. This device was linked into the tooth to be measured and into the spectrophotometer, allowing that this latter record always the same tooth area, in all performed measurements. The spectrophotometer provides the values L*, a* and b* coordinates CIE Lab, for each analyzed tooth. L* indicates the luminosity; a* and b* indicates the shade, where a* represents the color and saturation in the red-green axis and b* represents the blue-yellow axis. Shade comparison before and after tooth bleaching, for each time period, was obtained by the color difference (ΔE), by the following formula: ∆E ∆E =[(∆L)2+ (∆a)2+(∆b)2]1/2, in which ∆L = final L-initial L; ∆a = final a-initial a, and ∆b = final b-initial b. Data statistical comparison was performed by ANOVA and Bonferroni tests.
Results
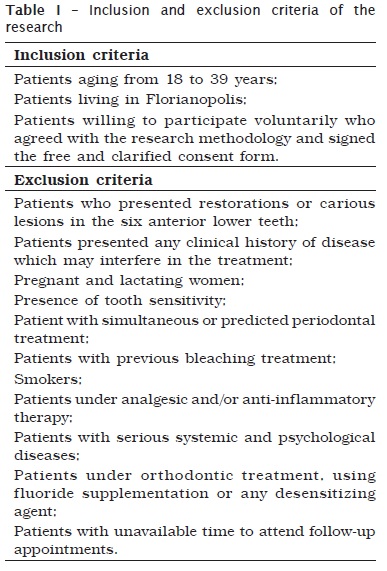
ANOVA for repeated measurements showed that there was a statistically significant difference in color change of teeth, over time, in both used methods of assessment (p-value < 0.001). Table II and graph 1 showed that 14-day ∆E ∆E was smaller and statistically different of 90-day and patient satisfaction ∆E ∆E.
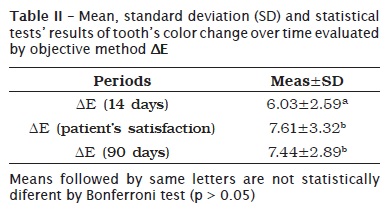
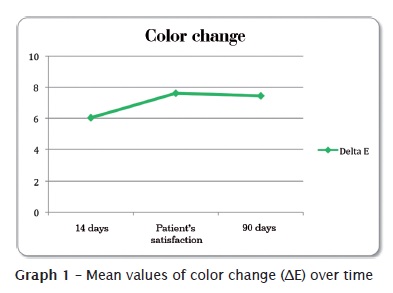
In table III and graph 2, we noted that tooth's initial color mean was 7.17, which means a color between C2 and D4 when Vita Classic Scale was ordered by value. After two weeks of bleaching treatment, there was a reduction of about 3 shades, resulting in a shade close to D2, which is statistically diferent from baseline values. Notwithstanding, patients were satisfied after 27 days of treatment, in average, when teeth reached a shade close to B2, which was statistically different from two-week values.

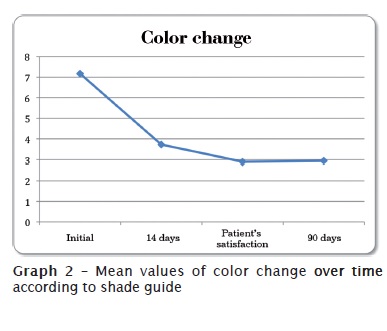
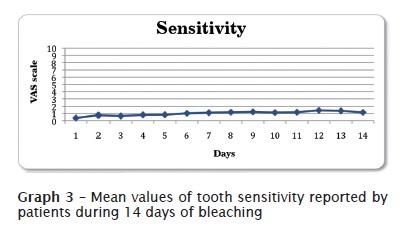
Graph 3 shows the results of tooth sensitivity reported by the patients during the first two weeks of treatment. Tooth sensitivity was slow: general mean of all results was equal to 1.01, in a scale ranging from 0 to 10.
Discussion
Clinical evaluations were the best way to verify bleaching agents' effectiveness because they include patients' individual factors (habits, food, hygiene, among others) which can influence on the final tooth shade. Therefore, because the research is conducted in a population with distinct life-style characteristics, the found bleaching degree tend to be more likely to the one obtained in clinical practice.
Among the methodologies used for evaluating tooth bleaching effectiveness in literature 15, the methods most commonly employed in clinical evaluations have been: comparison with visual standardized shade guide and intrumental messurement with spectrophotometers 22. The main advantage of visual scale is the low cost; however, because it is a subjective method, it could be influenced by patient's clothes and skin color as well as ilumination 8, examiner experience, and visual acuity. To minimize such factors, shade assessment was performed by one single examiner under 6,500 K light. Spectrophotometer measurements has a higher cost and is more complex; on the other hand, it is objetive, precise, and allows the numerical measurement of tooth color change in a tridimensional scale (CIELab) 7. This study results corroborate literature and demonstrate that both methodologies are effective in assess tooth color changes 7. Accordingly, both reseachers and dentists can use standardized shade guides to assess bleaching treatment effectiveness when a spectrophotometer is not available.
Our results corroborate literature 3,22,24 and proved that at-home bleaching is effective for vital bleaching teeth. Among the bleaching materials available for at-home bleaching technique, hydrogen and carbamide peroxide is highlighted. Regardless of the material type, hydrogen peroxide is the active agent which promotes tooth bleaching acting as oxidizing agent and reacting with the aromatic rings, breaking them into smaller and clearer molecules 13. Consequently, bleaching degree depends on hydrogen peroxide concentration, the ability of breaking aromatic ring chains, and frequency and time period that bleaching agent is in contact with macromolecules 7.
In our study, patient's satisfaction was reached at 27 days of treatment, in average. Two-week bleaching treatment promoted a clinically visible color change of the teeth (∆E ∆E greater than 3.3 units) 28; however, this value was not sufficient for pleasing 90% of the patients. Data of patient's satisfaction found by our study were in agreement with another clinical study: according to Bernardon et al. 2, patient's satisfaction was reached between 4 and 6 weeks of treatment, regardless of the bleaching agent or technique employed. Most of the studies used a pre-established time period to bleaching execution; however, in clinical practice is important to consider patient's opinion for establishing treatment length 7. In this study, we observed that there was a statistically significant difference between 14-day treatment and patient's satisfaction assessment, showing that pre-established periods can be used as reference both for the patient and the clinician. Notwithstanding, ideal treatment length should be discussed with the patient during follow-up appointments.
It is also noted that after 12 weeks tooth color was stable in comparison with the color obtained at the treatment ending (patient's satisfaction). This information indicates that bleaching treatment was effective and corroborates other clinical studies, which demonstrated that there is no significant regression of tooth color after bleaching treatment 3,7.
Tooth sensitivity has been reported as the main side effect of tooth bleaching, resulting in treatment abandonment 12. Literature has demonstrate that 15% to 65% of patients show tooth sensitivity increase during bleaching treatment 17. This increase occurs because hydrogen peroxide propagates through enamel and dentin, reaching the pulp 9,27. In our study, tooth sensitivity reported by the patients was low: 1.01 in a scale from 0 to 10. Mild tooth sensibility may be related to the presence of potassium nitrate, calcium and sodium fluoride in bleaching agent composition. According to Browning et al. 4, patients undergoing tooth bleaching with agents containing desensitizing showed a smaller sensitivity rate.
Since bleaching treatment should be conducted both in upper and lower arch, researches evaluating tooth sensitivity and bleaching agent effectiveness in both arches are of extreme importance because they help the dentist to execute clinical procedures based on scientific evidences. Therefore, further studies are necessary to compare upper and lower arch whitening degree, as well as the effects of different techniques, application time periods, and bleaching agents.
Conclusion
Our results showed that at-home bleaching technique with 4% hydrogen peroxide for 2 hours per day presents low sensitivity and it is effective. Patient's satisfaction was reached after 27 days of treatment, in average.
Acknowledgments
Authors thank FGM for providing the bleaching agents used in this research; the Brazilian Institutional Program of Scientific Initiation Scholarship by this project approval and the scholarship grant; and Sillas Duarte Júnior, DDS, for abstract editing.
References
1. Al-Salehi SK, Wood DJ, Hatton PV. The effect of 24h non-stop hydrogen peroxide concentration on bovine enamel and dentine mineral content and microhardness. J Dent. 2007 Nov;35(11):845-50. [ Links ]
2. Bernardon JK, Ferrari P, Vieira LCC, Maia HP. Avaliação do tempo de tratamento para a satisfação do paciente nas diferentes técnicas de clareamento. Braz Oral Res. 2010;24(Supplement 1):271.
3. Bernardon JK, Sartori N, Ballarin A, Perdigão J, Lopes GC, Baratieri LN. Clinical performance of vital bleaching techniques. Oper Dent. 2010 Jan-Feb;35(1):3-10.
4. Browning WD, Chan DC, Myers ML, Brackett WW, Brackett MG, Pashley DH. Comparison of traditional and low sensitivity whiteners. Oper Dent. 2008 Jul-Aug;33(4):379-85.
5. Browning WD. Use of shade guides for color measurement in tooth-bleaching studies. J Esthet Restor Dent. 2003;15(Suppl 1):S13-20.
6. Burrows S. A review of the efficacy of tooth bleaching. Dent Update. 2009 Nov;36(9):537-8, 541-4, 547-8.
7. Cardoso PC, Reis A, Loguercio A, Vieira LC, Baratieri LN. Clinical effectiveness and tooth sensitivity associated with different bleaching times for a 10 percent carbamide peroxide gel. J Am Dent Assoc. 2010 Oct;141(10):1213-20.
8. Chu SJ. Use of a reflectance spectrophotometer in evaluating shade change resulting from tooth-whitening products. J Esthet Restor Dent. 2003;15(Suppl 1):S42-8.
9. Dahl JE, Pallesen U. Tooth bleaching: a critical review of the biological aspects. Crit Rev Oral Biol Med. 2003;14(4):292-304.
10. Gladwell J, Simmons D, Wright JT. Remineralization potential of a fluoridated carbamide peroxide whitening gel. J Esthet Restor Dent. 2006;18(4):206-12; discussion 212-3.
11. Haywood VB, Heymann HO. Nightguard vital bleaching. Q Quintessence Int. 1989 Mar;20(3):173-6.
12. Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent. 2005 Sep;26(9 Suppl 3):11-20.
13. Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007 Dec;35(12):889-96.
14. Joiner A. The bleaching of teeth: a review of the literature. J Dent. 2006 Aug;34(7):412-9.
15. Joiner A. Tooth colour: a review of the literature. J Dent. 2004;32(Suppl 1):3-12.
16. Kihn PW. Vital tooth whitening. Dent Clin North Am. 2007 Apr;51(2):319-31.
17. Leonard Jr RH, Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital bleaching. Quintessence Int. 1997 Aug;28(8):527-34.
18. Maia E, Baratieri LN, Caldeira de Andrada MA, Monteiro Jr S, Vieira LC. The influence of two home-applied bleaching agents on enamel microhardness: an in situ study. J Dent. 2008 Jan;36(1):2-7.
19. Matis BA, Gaiao U, Blackman D, Schultz FA, Eckert GJ. In vivo degradation of bleaching gel used in whitening teeth. J Am Dent Assoc. 1999 Feb;130(2):227-35.
20. Mccracken MS, Haywood VB. Demineralization effects of 10 percent carbamide peroxide. J Dent. 1996 Nov;24(6):395-8.
21. Minoux M, Serfaty R. Vital tooth bleaching: biologic adverse effects – a review. Quintessence Int. 2008 Sep;39(8):645-59.
22. Myers ML, Browning WD, Downey MC, Hackman ST. Clinical evaluation of a 3% hydrogen peroxide tooth-whitening gel. J Esthet Restor Dent. 2003;15(1):50-5; discussion 56.
23. Pinto CF, Oliveira R, Cavalli V, Giannini M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz Oral Res. 2004 Oct-Dec;18(4):306-11.
24. Sarrett DC. Tooth whitening today. J Am Dent Assoc. 2002 Nov;133(11):1535-8.
25. Seymour RA. The use of pain scales in assessing the efficacy of analgesics in post-operative dental pain. Eur J Clin Pharmacol. 1982;23(5):441-4.
26. Swift Jr EJ, May Jr KN, Wilder Jr AD, Heymann HO, Bayne SC. Two-year clinical evaluation of tooth whitening using an at-home bleaching system. J Esthet Dent. 1999;11(1):36-42.
27. Thitinanthapan W, Satamanont P, Vongsavan N. In vitro penetration of the pulp chamber by three brands of carbamide peroxide. J Esthet Dent.1999;11(5):259-64.
28. Um CM, Ruyter IE. Staining of resin-based veneering materials with coffee and tea. Quintessence Int. 1991 May;22(5):377-86.
29. Zekonis R, Matis BA, Cochran MA, Al Shetri SE, Eckert GJ, Carlson TJ. Clinical evaluation of in-office and at-home bleaching treatments. Oper Dent. 2003 Mar-Apr;28(2):114-21.
 Correspondence:
Correspondence:
Universidade Federal de Santa Catarina
Campus Universitário Reitor João David Ferreira Lima – Trindade
Departamento de Odontologia
Disciplina de Dentística
CEP 88040-970 – Florianópolis – SC – Brasil
E-mail: ladape@gmail.com
Received for publication: January 24, 2011
Accepted for publication: April 12, 2011.













