Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.1 Joinville Jan./Mar. 2012
ORIGINAL RESEARCH ARTICLE
Antifungal activity of plant-based tinctures on Candidas
Andreia Medeiros Rodrigues Cardoso I; Yuri Wanderley Cavalcanti I; Leopoldina de Fátima Dantas de Almeida II; Ana Luíza Alves de Lima Pérez I; Wilton Wilney Nascimento Padilha III
I Undergraduate Student, School of Dentistry, Federal University of Paraiba – João Pessoa – PB – Brazil.
II Program of Post-Graduation in Dentistry (Preventive Pediatric Dentistry), Master Course in Dentistry, University of Paraiba – João Pessoa – PB – Brazil.
III Department of Clinics and Social Dentistry, University of Paraiba – João Pessoa – PB – Brazil.
ABSTRACT
Objective: To evaluate through determination of minimum inhibitory concentration (MIC) the antifungal activity of Salvia officinalis (sage), Anacardium occidentale (cashew) and Malva sylvestris (mallow) tinctures on Candida albicans (ATCC 40227), C. tropicalis (ATCC 13803) and C. krusei (ATCC 40147). Material and methods: In 96-well microplates, 100 μl of Sabouraud-Dextrose broth doubly concentrated, 100 μl of the tested tinctures and 10 μl of fungal inoculums (1.5 x 106 organisms/ml) were inserted. The products were diluted from initial concentration of 100 mg/ml until 0.78 mg/ml. MIC corresponded to the lowest dilution at which there was no visible fungal growth. Nystatin (100,000 UI/ml) was used as control. Statistical analysis was performed by Kruskal-Wallis and Dunn tests (p < 0.05). Results: S. officinalis tincture did not inhibit the growth of C. albicans and C. tropicalis; MIC was 100 mg/ml for C. krusei. For A. occidentale, MIC was 100 mg/ml for C. albicans and C. krusei, and for C. tropicalis, there was no fungal inhibition. M. sylvestris tincture presented MIC at 25 mg/ml for C. krusei and 100 mg/ml for C. albicans and C. tropicalis. The best antifungal activity was showed by M. sylvestris tincture (p < 0.05). Conclusion: M. sylvestris tincture exhibited antifungal activity against all the tested strains at lower concentrations. S. officinalis tincture inhibited the action of C. krusei and A. occidentale tincture showed activity against C. albicans and C. tropicalis.
Keywords: natural products; oral candidiasis; products with antimicrobial action.
Introduction
Oral candidiasis is one of the most common human infections of fungal nature. It has been described as an opportunistic infection, frequently involved with oral microbiota alteration, systemic diseases, and reduction of the host immunity 1,6. Among the strains implicate in oral candidiasis development, Candida albicans is the most prevalent and highest pathogenic microorganism 1,16. C. tropicalis, C. krusei, C. parapsilosis and C. guilliermondii are also present in the disease course and together with C. albicans represent more than 80% of the clinical isolates 1,16.
Stramandinoli et al. 23 (2010) evaluate the main risk factors for oral candidiasis prevalence in hospitalized patients. It was identified that the use of nasogastric tube, prostheses, and poor oral hygiene represented a serious risk to the development of this infection. Several topic and systemic antifungals have been used for candidiasis treatment, according to the patient's clinical and general state 3.
According to Alves et al. 3 (2009), Nystatin is the drug of choice for topic treatment of C. albicans infections within oral cavity, however, microbial resistance is a growing problem. For the systemic treatment of patients at high risk of developing fungal infections, literature has recommended the use of fluconazole.
Rex et al. 19 (2000) and Khan et al. 8 (2009) affirmed that C. albicans strains have become resistant due to the use of some synthetic antifungal drugs. Aiming to identify substitutes to the traditional drugs, studies on the antimicrobial activity of natural products have been conducted 5,18. According to Alves et al. 3 (2009), these products present antimicrobial and anti-inflammatory properties, relatively low cost, and inhibitory activity against resistant microorganisms. Therefore, the search for natural products presenting an efficient antifungal action against resistant microorganism is a necessary alternative for controlling oral candidiasis 5,18. In this context, Maekawa et al. 10 (2007) demonstrated that the use of herbal substances, e.g. chlorophyll extract, may be effective to control C. albicans.
Ethnobotanical researches identified the popular use of Salvia officinalis (sage), Anacardium occidentale (cashew) and Malva sylvestris (mallow) 7,21, justifying this study execution. Also, the antimicrobial activity of Salvia officinalis (sage), Anacardium occidentale (cashew) and Malva sylvestris (mallow) natural extracts has been described by several studies 3,11,12,13,17,18.
On the other hand, there are few reports on these products' inhibitory concentration. Consequently, it is mandatory to investigate their antifungal activity, aiming to justify and validate their use. Therefore, the aim of this study was to evaluate the in vitro antifungal activity of Salvia officinalis (sage), Anacardium occidentale (cashew) and Malva sylvestris (mallow) tinctures on C. albicans strains.
Material and methods
We conducted a study with an inductive approach, a comparative procedure and direct documentation technique in laboratory 9.
For in vitro antifungal assessment, Salvia officinalis (sage), Anacardium occidentale (cashew) and Malva sylvestris (mallow) tinctures (Farmácia Homeopática Homeovitae Ltd., João Pessoa, PB, Brazil) were used. These were obtained at concentration of 20% (200 mg/ml) and density of about 0.9 g/ml.
This study's reference strains were C. albicans (ATCC 40227), C. tropicalis (ATCC 13803) and C. krusei (ATCC 40147), obtained from the Laboratory of Reference Materials of the National Institute of Quality Control in Health (Oswaldo Cruz Foundation – FIOCRUZ –, Rio de Janeiro, RJ, Brazil). The strains were reactivated in Sabouraud-Dextrose broth (DIFCO, Detroit, Michigan, EUA) at 37ºC, and stored in Sabouraud-Dextrose agar 4% (DIFCO, Detroit, Michigan, EUA) at the Laboratory of Oral Microbiology, Nucleus of Tropical Medicine, Center of Health Sciences, Federal University of Paraiba. To conduct the study, fungal suspensions were prepared in sterile saline (0.85% NaCl), at concentration of 1.5x106 microorganisms/ml, equivalent to 106 MacFarland scale tube.
The tinctures' antifungal activity was evaluated by determining the minimum inhibitory concentration (MIC). For this purpose, the technique of microdilution described by Aligiannis et al. 2 (2001) and Castro & Lima 5 (2010) was employed. We use 96-well microplates (Alamar, Diadema, SP, Brazil), disposed in 12 columns (1 to 12) and eight lines (A to H). Each one of these plates was intended to analyze one microorganism. Columns 1, 2, and 3 were used for the antifungal analysis of Salvia officinalis tincture; columns 4, 5, and 6 for Anacardium occidentale tincture; and columns 7, 8, 9 for Malva sylvestris tincture. Column 10 was used for growth control; column 11 for sterility control and column 12 for positive control (Nystatin 100,000 UI/ml).
In each one of the microplates' well, 100 μl Sabouraud-Dextrose broth doubly concentrated were inserted. Following, 100 μl of the tested tinctures were inserted to obtain the initial concentration of 100 mg/ml in the first line of the microdilution plate 2,5. The following concentrations of the tinctures were obtained after the products' serial dilution in the microdilution plate, from 100 mg/ml (line A) until 0.78 mg/ml (line H), through transferring 100 μl of the content to the next well 2. In line H's wells, 100 μl of the content was dispensed to level the total volume of the wells.
Next, 10 μl of the fungal suspension (1.5 x 106 microorganisms/ml) were inserted in all wells, except from those corresponding to the sterility control column. Plates were incubated in bacteriological incubator at 37ºC, for 48h. MIC corresponded to the last tinctures concentration in which was not verified the presence of fungal precipitate or culture medium turbidity after the incubation period 2,5.
Each natural product's antifungal activity was assessed three times (triplicate) against each C. albicans strain. It was performed three samples for each condition and one reading for each sample (n = 27). This study's evaluated concentrations were pre-established and it was expected that the same products would exhibit the same MIC, when the triplicate was performed. Therefore, data was statistically analysed by Kruskal-Wallis and Dunn tests, with level of confidence set at 95%.
Results
The methodology employed in this study was validated by the lack of fungal growth for the sterility and positive control (Nystatin 100,000 UI/ml), as well as by the presence of fungal growth for the growth control. Statistical differences and MIC values of the products tested against Candida strains are shown in table I.
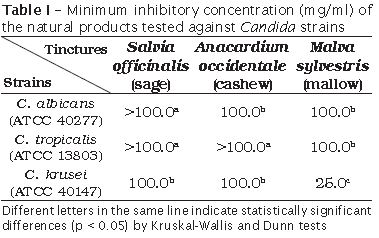
At the tested concentrations, we verified that S. officinalis tincture did not exhibited activity against C. albicans and C. tropicalis. A. occidentale tincture did not inhibit the activity of C. tropicalis. Statistically significant difference (p < 0.05) was found for the inhibitory effect of M. sylvestris tincture (against C.tropicalis and C. albicans) and A. occidentale tincture (against C. albicans). M. sylvestris tincture exhibited antifungal activity against all the tested strains at lower concentrations with statistically significant difference (p < 0.05).
Discussion
The reference method for the microdilution technique and determination of the yeast sensitivity to antifungal therapy (M7-A2), described by the National Committee for Clinical Laboratory Standards (NCCLS) 15 (2002), considers the evaluation of synthetic antifungals, the use of the culture medium RPMI-1640 and the standardization of the initial concentration of the tested substances at 64 μg/ml or 16 μg/ml. The technique for determining the MIC employed in this study was based on the protocols described by Aligiannis et al. 2 (2001) and Castro & Lima 5 (2010). These protocols represent the modification of the regulations established by NCCLS 15 (2002).
This present study was developed at different conditions from those established by NCCLS 15 (2002). According to Nascimento et al. 14 (2007), the regulation M7-A2 proposed by NCCLS could not be followed to the letter when performing the antimicrobial evaluation of the plant tinctures. It is considered that the chemical properties of these natural products are different from those presented by the substances, for which the regulation was standardized, i.e., NCCLS Regulation does not meet the specifications of non-synthetic products. Therefore, we reproduced the protocol described by Aligiannis et al. 2 (2001) and by Castro & Lima 5 (2010), in which they employed the Sabouraud-Dextrose broth as culture medium, Nystatin as positive control, and the serial dilution of the tested products. Because this study's tinctures are hydroalcoholic solutions (hydrophilic), the addition of the emulsifier (Tween 80) in the antifungal evaluation of these products is not necessary.
By comparing the techniques of disk-diffusion and microdilution to evaluate the antifungal activity of natural products, Scorzoni et al. 20 (2007) identified that microdilution was more sensitive for MIC determination. Therefore, to diminish the inconsistency of the obtained results, we employed the broth microdilution technique, aiming to enable a greater contact between the tested products and the fungal cells. Additionally to these factors, the incorporation of the positive, sterility, and growth control contributes to both the reduction of the methodological bias and the comparison among the tested natural products 5,20.
The sterility, growth, and positive controls were used to validate the technique employed in this study. The sterility and growth controls proved, respectively, the lack of culture medium contamination and viability of the tested strains. The absence of fungal growth against Nystatin (100,000 UI/ml) demonstrates the samples' susceptibility against the synthetic antifungal. Due to the differences between the chemical nature of the natural products and of the positive control, it was not possible to compare the minimum inhibitory concentrations obtained against the tested microorganisms.
According to Nascimento et al. 14 (2007), the natural products exhibit greater antimicrobial activity within the formulation of essential oils. This would be justified by the higher concentration of active principles and the lipidic nature of the essential oil. The liposoluble nature of essential oils and its components enables the interaction with the lipidic cellular structures, resulting in the increase of the membranes' permeability, which can provoke electrolyte imbalance and cellular death 5,14. However, the tinctures tested in this study showed a final concentration of 20% due to chemical processes of dilution in ethilic alcohol (hydrosoluble characteristic). Considering the commercial availability of natural extracts for therapeutic use, literature has considered that natural product tinctures exhibit lower antimicrobial activity and active principles' concentration than essential oils 14.
Pozzatti et al. 18 (2009) evaluated the antifungal activity of S. officinalis against clinical isolates of C. albicans and C. dubliniensis. The authors verified that this natural product did not inhibit the microorganism growth at concentrations lower than 3.2 mg/ml. By evaluating C. albicans sensitivity to S. officinalis extract, Nascimento et al. 13 (2000) did not identified this product's antifungal activity. The results of this study corroborate the literature findings by demonstrating the lack of antifungal activity of S. officinalis tincture at concentrations lower than 100 mg/ml against C. albicans and C. tropicalis.
Molina et al. 12 (2008) evaluated the antifungal activity of Salvia officinalis glycolic extract by broth dilution method on 20 strains of C. albicans isolated from the oral cavity. The authors identified that the natural product inhibited the fungal activity in 80% of the tested strains 12. However, this study's results pointed out that among the microorganisms tested only C. krusei showed growth inhibition against S. officinalis at 100 mg/ml. Therefore, further studies are necessary to refute or confirm the antifungal activity of this natural product.
We did not identify studies on the antifungal activity of A. occidentale on C. albicans. Considering the antibacterial activity of this natural product, literature shows the inhibition of the microorganisms involved in the cariogenic biofilm formation 17 and involved with hospital infections 22. Pereira et al. 17 (2006) and Silva et al. 22 (2007) considered that polyphenols, tannin, and flavonoids within these products' composition account for the antimicrobial activity of A. occidentale. Our results demonstrated C. albicans and C. krusei growth inhibition at the concentration of 100 mg/ml. Therefore, there is a need for further studies on the antifungal activity of A. occidentale derivatives to confirm these products' actions.
The antimicrobial activity of the natural products based on M. sylvestris was researched by several studies 3,4,11, highlighting the clinical use of this product in mouthrinse formulations. Matos et al. 11 (2009) evaluated the antifungal activity of mouthrinses based on chlorhexidine gluconate (0.12%), hydrogen peroxide (1.5%), and M. sylvestris tincture on C. albicans strains. Although M. sylvestris tincture presented an antifungal activity against 73% of the strains, the solution exhibited lower effectivity than chlorhexidine and hydrogen peroxide. Our study found antifungal activity of M. sylvestris tincture at the concentrations of 100 and 25 mg/ml, which corroborates the findings of Alves et al. 3 (2009). When compared to the other products evaluated by our study, it was observed that M. sylvestris tincture exhibited a higher antifungal activity than S. officinalis and A. occidentale tinctures.
The methodology employed in this study demonstrated the antifungal activity of the tested products, highlighting the action of M. sylvestris tincture against the tested strains. Further studies on these products' antifungal activity through standardized and validated techniques are necessary. These should evaluate the antifungal activity against clinical strains and other standardized strains, employing techniques considering the phytoconstituent isolation, cellular adherence, biofilm formation in vitro, the influence of the human saliva and these products' toxicology.
Conclusion
Within this study's conditions, it can be concluded that M. sylvestris tincture presented antifungal activity against all tested strains, at lower concentrations. S. officinalis tincture inhibited the activity of C. krusei, and A. occidentale tincture exhibited antifungal activity against C. krusei e C. albicans.
References
1. Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002 Feb;78(2):455-9. [ Links ]
2. Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou JB. Composition and antimicrobial activity of the essential oils of two origanum species. J Agric Food Chem. 2001 Sep;49(9):4168-70.
3. Alves PM, Queiroz LMG, Pereira JV, Pereira MSV. Atividade antimicrobiana, antiaderente e antifúngica in vitro de plantas medicinais brasileiras sobre microrganismos do biofilme dental e cepas do gênero Candida. Rev Soc Bras Med Trop. 2009 Apr-Jun;42(2):222-4.
4. Candido RC, Azevedo RVP, Ito IY. Determination of the minimal inhibitory concentrations of Cepacol, Malvona and Periogard in Candida albicans strains isolated from oral cavity. Rev Odontol Unesp. 1996 Jan-Feb;25(1):79-84.
5. Castro RD, Lima EO. Atividade antifúngica in vitro do óleo essencial de Eucalyptus globulus L. sobre Candida spp. Rev Odontol Unesp. 2010 May-Jun;39(3):179-84.
6. Corrêa EM, Andrade ED. Tratamento odontológico em pacientes HIV/AIDS. Rev Odonto Ciênc. 2006 Jul-Sep;20(49):281-9.
7. Fenner R, Betti AH, Mentz LA, Rates SMK. Plantas utilizadas na medicina popular brasileira com potencial atividade antifúngica. Rev Bras Ciênc Farm. 2006 Jul-Sep;42(3):369-93.
8. Khan R, Islam B, Akram M, Shakil S, Ahmad AA, Ali SM et al. Antimicrobial activity of five herbal extracts against multi drug resistant (MRD) strains of bacteria and fungus of clinical origin. Mol Cells. 2009 Feb;14(2):586-97.
9. Lakatos EM, Marconi MA. Fundamentos da metodologia científica. 6. ed. São Paulo: Atlas; 2009. 379 p.
10. Maekawa LE, Lamping R, Marcacci S, Maekawa MY, Nassri MRG, Koga-Ito CY. Antimicrobial activity of chlorophyll-based solution on Candida albicans and Enterococcus faecalis. RSBO. 2007 Nov;4(2):36-40.
11. Matos BM, Deco CP, Oliveira LD, Jorge AOC, Balducci I, Koga-Ito CY. Comparação da atividade antimicrobiana de soluções de peróxido de hidrogênio e malva sobre Candida albicans. Ciênc Odontol Bras. 2009 Apr-Jun;12(2):24-8.
12. Molina FP, Majewski M, Perrela FA, Oliveira LD, Junqueira JC, Jorge AOC. Própolis, sálvia, calêndula e mamona – atividade antifúngica de extratos naturais sobre cepas de Candida albicans. Ciênc Odontol Bras. 2008 Apr-Jun;11(2):86-93.
13. Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000 Jul-Aug;31(4):247-56.
14. Nascimento PFC, Nascimento ALC, Rodrigues CS, Antoniolli AR, Santos PO, Barbosa-Júnior AM et al. Atividade antimicrobiana dos óleos essenciais: uma abordagem multifatorial dos métodos. Rev Bras Farmacogn. 2007 Jan-Mar;17(1):108-13.
15. National Committee for Clinical Laboratory Standards (NCCLS). Método de referência para testes de diluição em caldo para a determinação da sensibilidade a terapia antifúngica das leveduras. Norma M27-A2 do NCCLS. Pensilvânia: NCCLS; 2002. 51 p.
16. Nikawa H, Nishimura H, Hamada T, Yamashiro H, Samaranayake LP. Effects of modified pellicles on Candida biofilm formation on acrylic surfaces. Mycoses. 1999 Jan;42(1):37-40.
17. Pereira JV, Sampaio FC, Pereira MSV, Melo AFM, Higino JS, Carvalho AAT. In vitro anti microbial activity of an extract from Anacardium occidentale Linn. on Streptococcus mitis, Streptococcus mutans and Streptococcus sanguis. Odontol Clín Cient. 2006 May-Jun;5(2):137-41.
18. Pozzatti P, Loreto ES, Lopes PGM, Athayde ML, Santurio JM, Alves SH. Comparison of the susceptibilities of clinical isolates of Candida albicans and Candida dubliniensis to essential oils. Mycoses. 2009 Jan;53(1):12-5.
19. Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE et al. Practice guidelines for the treatment of candidiasis. J Infect Dis. 2000 Apr;30(4):662-78.
20. Scorzoni L, Benaducci T, Almeida AMF, Silva DHS, Bolzani VS, Mendes-Giannini MJS. Comparative study of disk diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp and Cryptococcus sp. Rev Ciênc Farm Básica Apl. 2007 Jan-Mar;28(1):25-34.
21. Silva MD, Dreveck S, Zeni ALB. Estudo etnobotânico de plantas medicinais utilizadas pela população rural no entorno do Parque Nacional da Serra do Itajaí – Indaial. Health Environ J. 2009 Feb;10(2):54-64.
22. Silva JG, Souza IA, Higino JS, Siqueira-Junior JP, Pereira JV, Pereira MSV. Atividade antimicrobiana do extrato do Anacardium occidentale Linn. em amostras multirresistentes de Staphylococcus aureus. Braz J Pharmacognosy. 2007 Oct-Dec;17(4):572-7.
23. Stramandinoli RT, Souza PHC, Westphalen FH, Bisinelli JC, Ignácio SA, Yurgel LS. Prevalência de candidose bucal em pacientes hospitalizados e avaliação dos fatores de risco. RSBO. 2010 Mar;7(1):66-72.
 Correspondence:
Correspondence:
Yuri Wanderley Cavalcanti
Avenida Des. Hilton Souto Maior, n.º 6.701, quadra 765, lote 117 – Portal do Sol
CEP 58046-600 – João Pessoa – PB – Bral
E-mail: yuri.wanderley@yahoo.com.br
Received for publication: March 1, 2011
Accepted for publication: June 27, 2011













