Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.1 Joinville Jan./Mar. 2012
ORIGINAL RESEARCH ARTICLE
Microbiological evaluation of infected root canals and their correlation with pain
Nadine Luísa Soares de Lima Guimarães I; Hanna Machado Otoch I ; Larissa Cavalcante de Andrade I ; Cláudio Maniglia Ferreira I ; Márcia Maria de Negreiros Pinto Rocha II ; Fábio de Almeida Gomes I
I Department of Endodontics, University of Fortaleza – Fortaleza – CE – Brazil.
II Department of Microbiology, University of Fortaleza – Fortaleza – CE – Brazil.
ABSTRACT
Introduction and Objective: The aim of this study was to evaluate the endodontic microbiota of human teeth without pulp vitality presenting radiographically visible periapical lesions and its correlation with pre- and postoperative pain symptomatology. Material and methods: Sixteen young adult patients, both genders, aging from 18 to 45 years, presenting 21 single-rooted teeth with pulp necrosis and needing endodontic treatment were selected in the multidisciplinary clinic at the University of Fortaleza (UNIFOR). After crown surgical access, the root canals were embedded with 0.9% saline solution and the material from root canals was collected with sterile paper point. The paper points were placed into Stuart transport medium and sent to the microbiology laboratory of the University of Fortaleza. Isolation and identification of bacteria were made by culture technique. The cleaning and shaping of root canals was performed by crown-down technique. Intra-canal medication comprised calcium hydroxide mixed with chlorhexidine and after 14 days the canals were filled. Patients were asked about the occurrence of pain before treatment and 24 hours after cleaning and shaping procedures. Results: The most prevalent microbial group was Streptococcus sp. followed by Fusobacterium nucleatum, although Gram-positive cocci, non-sporulating Gram-positive bacilli, Gram-negative bacilli, pigmented Gram-negative bacilli, Veillonella, Staphylococcus aureus, Pseudomonas sp. were also frequently isolated. Conclusion: According to the results, it can be concluded that Fusobacterium nucleatum and Gram-negative bacilli were more related to pre-operative pain. Regarding to postoperative pain, the most prevalent bacteria were Gram-positive cocci.
Keywords: microbiology; root canal; pain.
Introduction
Currently, it is understood that endodontic treatment success is directly associated with several factors relating to each other as links of a chain. If one of these links is broken, treatment success probability will significantly decrease. Among these factors, we can mention the accurate diagnosis, maintenance of the aseptic chain, knowledge on tooth anatomy, correct chemical-mechanical preparation, tridimensional obturation of root canal system, proservation, and when necessary, the use of intracanal medication. All these factors converge to a crucial point that is root canal system decontamination.
Microorganisms and their products represent the primary etiological agents of both pulp necrosis and of periapical lesions development 24,28,32. Their elimination is of fundamental importance to obtain root canal treatment success. Therefore, the lack of success is directly related to the difficulty of controlling and eliminating the endodontic infection 20.
From the 19th century to the middle of the 20th century, in teeth with pulp necrosis, the microbial species were isolated from root canal by predominantly aerobe bacterial culture techniques. The techniques were limited to the culture and identification of strict aerobes 1. Consequently, endodontic microbiota from teeth without pulp vitality was considered as predominantly aerobe and facultative 6,39,42. In the 70s, the progress in anaerobe culture techniques resulted in the isolation and culture of strict anaerobes recovered from necrotic root canals 29, and the next studies demonstrated the incidence of root canal infection by anaerobes above 90% 16,22,38,40,43.
Anaerobes have been related as the main cause of symptomatic cases, also being correlated to edema, pain, and odor 40.
Sundqvist et al. 40 observed that the number of bacterial species were greater in cases in which there were clinical manifestations as pain, edema, abscess, or fistula. Probably, at the initial phase, when the pain is more characteristic and the defense system is still mobilizing itself, the infection is being organized regarding to the influence of environmental determinants. The tendency is that the interaction among the microorganisms limits the number of species within root canal system, which would explain the fact the Sundqvist et al. 40 found a smaller number of species in chronic apical periodontitis.
It is worth noting, however, that isolated bacteria are different from patient to patient, i.e., bacteroids, Fusobacterium, Peptostreptococcus, Peptococcus and Eubacterium have been considered the primary etiological agents of several cases 21,38,40.
By taking into consideration the variety of the microbiota from root canals with pulp necrosis and its correlation with pain, the aim of this study was to evaluate the microbiota recovered from teeth without pulp vitality and with radiographically visible periapical lesion and its correlation with pre- and postoperative pain.
Material and methods
This transversal study aimed to evaluate the endodontic microbiota recovered from human teeth without pulp vitality presenting radiographically visible periapical lesion and its relation with painful symptomatology. Prior to the execution of the procedures, the project was approved by the Ethical Committee in Research of the University of Fortaleza – Unifor (328/2010).
Sample obtainment and selection
Sixteen healthy young adult patients, both genders, aging from 18 to 45 years, presenting 21 single-rooted teeth with pulp necrosis and needing endodontic treatment were selected in the multidisciplinary clinic at the University of Fortaleza (UNIFOR), from May to November, 2010.
Each selected tooth exhibited one single root canal and radiographic image suggestive of periapical lesion. All teeth were previously radiographed to confirm this study's inclusion criteria.
Exclusion criteria comprised the presence of systemic alteration, teeth with previous endodontic treatment, root canals exposed to oral environment, roots with radiographic image suggestive of transversal or longitudinal fracture, and teeth presenting periodontal problems or extensive carious lesions making their restoration impossible.
Material collection from root canal
All access, cleaning, shaping, and obturation of root canals were executed by a single operator.
After the crown's surgical access, root canal was copiously irrigated with 0.9% saline solution (Química Farmacêutica Gaspar Viana S.A., Fortaleza, CE, Brazil). Following, a sterile paper point (Tanarian Industrial Ltda., Manacapuru, AM, Brazil) with size compatible with root canal's anatomic diameter (AD) was inserted for 30 seconds to collect the material from root canal. After the paper point removal, this was placed into Stuart transport medium which preserves strict anaerobes for 40 minutes and facultative anaerobes for until 2 hours.
The tubes containing the transport medium were identified with labels exhibiting the patient's name and tooth and the day of the collection. Also, an individual record was filled in with information of each collection, before the material was sent to the microbiology laboratory of the University of Fortaleza.
Bacterial identification
For the isolation and identification of the strict anaerobes, the following culture mediums were used: Brain heart infusion agar (Difco) supplemented with defibrinated sheep blood, hemin (Sigma) and menadione (Biochemical); and Brain heart infusion broth (Difco) supplemented with hemin and menadione.
The plates with culture mediums were immediately stored in anaerobic jar containing the gas mixture (80% N2; 10% CO2; and 10% H2) and incubated at 37oC for five to seven days. After that period, the bacterial colonies were examined by stereoscope microscopy and characterized. All morphologically distinct colonies were subcultured into broth culture mediums (BHI) and incubated at 37oC for 24 to 48 hours. The respiratory metabolism test was performed. The different colony smears were executed by Gram method. After the assessment of the purity of broth cultures, the morpho-tinctorial characterization, respiratory metabolism test, and biochemical proves were executed, such as: catalase, fermentation (lactose) and coagulase tests for genus characterization, and whenever possible, for isolated bacterial species' characterization, following the methodology described by Murray et al. 30.
Parallely to the strict anaerobes' culture, the clinical specimen was seeded in BHI broth, BHI agar supplemented with defibrinated sheep blood and MacConkey agar (Difco) to isolate the facultative anaerobes. BHI broth and agar were stored in microaerophilic jar (candle method), while MacConkey agar was maintained into conventional atmosphere at 37oC for 24 to 48 hours. To characterize the genus and the specie, biochemical tests were executed following the methodology described by Murray et al. 30.
Pain versus bacterial involvement
After obtaining the results, we correlated pre- and postoperative pain presence or absence with the bacteria found in each analysed case. The pain presence was assessed at two distinct moments: prior to treatment: the patient was asked about pain occurrence at any time before the treatment onset; and post-treatment: 24 hours after root canal's instrumentation. Through a careful clinical evaluation, we discarded the possibility that the pre-operatory pain reported by the patient had initiated in another tooth.
Results
One out of the 21 samples was discarded due to technical problems. Consequently, this study's final sample comprised 20 root canals of 16 patients. Prior to the endodontic access, two teeth exhibited fistula; seven patients reported pain, and from these, 2 showed edema. The left 11 teeth did not exhibit painful symptomatology or any other symptom.
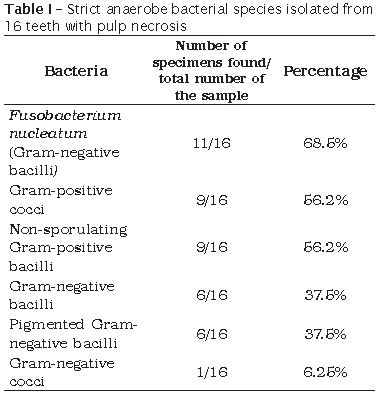
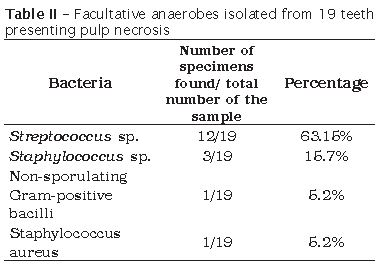
We obtained 16 samples of strict anaerobes from those isolated after the endodontic access and before root canal's chemical-mechanical preparation, because four specimens underwent contamination. Fusobacterium nucleatum (Gram-negative bacilli), Gram-positive cocci and non-sporulating Gram-positive bacilli were the most found bacteria (table I). Concerning to facultative anaerobes, 19 samples were obtained because one did not exhibit any growth. Streptococcus sp. was the most isolated bacteria, followed by Staphylococcus sp. and Staphylococcus aureus (table II).
In our study, two strict anaerobe species of Pseudomonas genera was isolated.
Results
The methodology employed in this study was validated by the lack of fungal growth for the sterility and positive control (Nystatin 100,000 UI/ml), as well as by the presence of fungal growth for the growth control. Statistical differences and MIC values of the products tested against Candida strains are shown in table I.
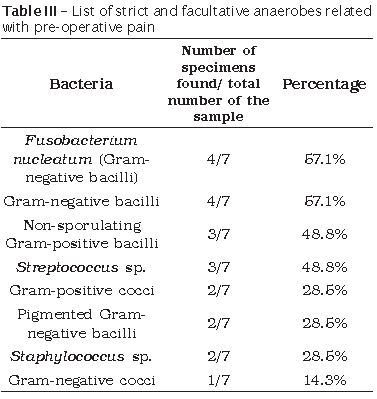
Seven out of 20 analysed teeth presented pre-operative pain symptomatology. In these cases, among the strict anaerobes Fusobacterium nucleatum together with other Gram-negative anaerobic bacilli were the most frequent followed by non-sporulating Gram-positive bacilli, Gram-positive cocci, pigmented Gram-negative bacilli, and Gram-negative cocci. Regarding to the facultative anaerobes, Streptococcus sp. was the most frequent bacteria, followed by Staphylococcus sp. (table III).
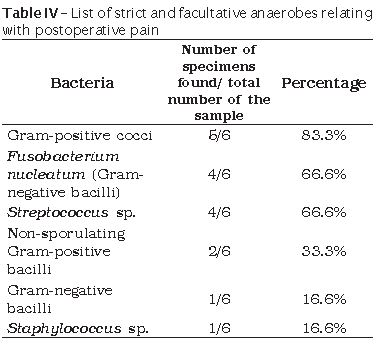
Six patients exhibited post-operative pain. Concerning to the strict anaerobes, Gram-positive cocci were the most frequent, followed by Fusobacterium nucleatum, non-sporulating Gram-positive bacilli, pigmented Gram-negative bacilli, and Gram-negative bacilli. By relating the postoperative pain with facultative anaerobes, Streptococcus sp. was the most prevalent, followed by Staphylococcus sp. (table IV).
Discussion
Several studies have been conducted to evaluate the endodontic microbiota 5,7,13,18,33,35,36 in different clinical and histopathological conditions (pulp necrosis, acute and chronic dentoalveolar abscess, granulomas and periapical cysts, endodontic retreatments, parendodontic surgeries), establishing correlations between the present signs / symptoms and the detected microorganisms 17,19,31,37.
In all cases with pulp necrosis, endodontic infection control plays the essential role in obtaining endodontic treatment success. In the beginning of the infectious process within the pulp tissue, it is observed the prevalence of a Gram-positive microbiota composed mainly of facultative aerobes, with cocci predominance over bacilli and filamentous microorganisms 14. However, since the 80s, due to the advancement in bacterial culture and identification techniques, it has been evidenced that root canals of teeth presenting pulp necrosis and radiographically visible chronic periapical lesion have predominantly strict anaerobes 14,18, particularly gram-negative microorganisms 2.
In this present study, the most prevalent microbial group in the clinical specimens collected prior to the biomechanical preparation was Streptococcus sp., followed by Fusobacterium nucleatum, although Gram-positive cocci, non-sporulating Gram-positive bacilli, Gram-negative bacilli, pigmented Gram-negative bacilli, Veillonella, Staphylococcus aureus, Pseudomonas sp. also were frequently isolated.
Rocha et al. 34 found bacteria in periapical lesions similar to those found within the root canal lumens of this study. As an example, it can be cited: Fusobacterium nucleatum, pigmented Gram-negative strict anaerobe bacilli, and Peptostreptococcus sp.
Among the recovered bacterial species in our study, facultative anaerobes were seen in 95% of the sample; Streptococcus sp. was present in 63.15%. On the other hand, Alencar et al. 1 found 100% of their cases with growth of facultative anaerobes and 83% of Streptococcus sp.
You et al. 41 reported that Staphylococcus aureus has close relationship with endodontic infections, particularly with piogenic cases; however, our results showed the presence of these bacteria in only 5.2% of the cases.
Concerning to strict anaerobes, black-pigmented bacilli (Porphyromonas sp. and Prevotella sp.) have been associated to signs and symptoms of endodontic origin, such as: spontaneous pain, pain to percussion, pain to palpation, swelling, and presence of exsudate 3,18,23. Our results showed that these bacteria were present in 37.5% of the cases and were related with pre- and postoperative pain in 28.5% and 16.6% of the cases, respectively. Still regarding to the cases with painful symptomatology, Fusobacterium nucleatum was the most frequent bacterium in cases with pre- (57.1%) and postoperative (66.6%) pain.
Although we did not observe growth of Enterococcus faecalis, these bacteria are of great importance in Endodontics because they have been associated with chronic periapical lesions and persistent infections within root canals; additionally, they are microorganisms resistant to unfavorable environmental conditions and, consequently of difficult elimination 9.
Despite of the great number of anaerobes isolated by our study, it is possible that some species very sensitive to oxygen presence have not been identified, even with all the caution during the method execution. Samples were inoculated into an appropriate transport medium and next into highly-nutritive, pre-reduced culture medium. This latter was readily incubate for enough time periods, under adequate gas conditions, to avoid the loss of slow-growth microorganisms.
Prior to the 70s, the bacterial species most recovered from root canals were Streptococcus viridans, facultative and gram-positive microorganisms, because the culture techniques were executed only in aerobiosis. Posteriorly, pre-reduced culture mediums were developed to be applied in medical and subsequently oral microbiology. Berg and Nord 4, by employing a mixture of 3% of H2 and N2, recovered 50% more anaerobes compared to the conventional technique. From that moment on, high percentage of obligatory anaerobes has been recovered from root canals, with mean of 79.9%.
On one hand, the culture method is not able to detect microorganisms with difficult growth; on the other hand, it is the only method capable of identifying exclusively the viable microorganisms. Several authors have used this technique to identify the microorganisms seen in endodontic infections 11,12,15,18,25,26,39,40,42. Microbiologic culture is the gold standard in the identification of the microbiota associated with several infectious diseases, including the endodontic infections.
Conclusion
• Among the strict anaerobes, Fusobacterium nucleatum was the most frequent;• Streptococcus sp. was the most prevalent facultative anaerobe in this study;
• The strict anaerobes most related with preoperative pain were Fusobacterium nucleatum and gram-negative bacilli. Regarding to facultative anaerobes, Streptococcus sp. was the most found, followed by Staphylococcus sp.
• Among strict anaerobes, gram-positive cocci were the most prevalent bacteria associated with postoperative pain. Streptococcus sp. was the facultative anaerobe most prevalent microorganism in cases of postoperative pain.
References
1. Alencar AH, Pimenta FC, Ito IY, Bruno KF, Leonardo MR. Determinação dos microrganismos no canal radicular, antes do preparo biomecânico e após a utilização da medicação intracanal, em dentes com necrose pulpar e reação periapical crônica. Arq Odontol. 2005;41(2):105-92. [ Links ]
2. Assed S, Ito IY, Leonardo MR, Silva LAB, Lopatin D. Anaerobic microorganisms in root canals of human teeth with chronic apical periodontitis detected by indirect immunofluorescence. Endo Dent Traumatol. 1996;12(2):66-9.
3. Baumgartner JC, Watkins BJ, Bae KS, Xia T. Association of black-pigmented bacteria with endodontic infections. J Endod. 1999;25(6):413-5.
4. Berg JO, Nord CE. A method for isolation of anaerobic bacteria from endodontic specimens. Scand J Dent Res. 1973;81(2):163-6.
5. Blome B, Braun A, Sobarzo V, Jepsen S. Molecular identification and quantification of bacteria from endodontic infections using real-time polymerase chain reaction. Oral Microbiol Immunol. 2008;23(5):384-90.
6. Brown Jr. LR, Rudolph Jr. CE. Isolation and identification of microorganisms from unexposed canals of pulp-involved teeth. Oral Surg. 1957;10(10):1094-9.
7. Bruno KF, Alencar AHG, Estrela C, Batista AC, Pimenta FC. Microbiological and microscopic analysis of the pulp of non-vital traumatized teeth with intact crowns. J Appl Oral Sci. 2009;17(5):508-14.
8. Claffey E, Reader A, Nusstein J, Beck M, Weaver J. Anesthetic efficacy of articaine for inferior alveolar nerve blocks in patient with irreversible pulpitis. J Endod. 2004;30(8):568-71.
9. Dahlén G, Samuelson W, Molander A, Reit C. Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral Microbiol Immunol. 2000;15(5):309-12.
10. De Deus QD. Endodontia. 5. ed. Rio de Janeiro: Medsi; 1992.
11. Dougherty WJ, Bae KS, Watkins BJ, Baumgartner JC. Black-pigmented bacteria in coronal and apical segments of infected root canals. J Endod. 1998;24(5):356-8.
12. Drucker DB, Gomes BPFA, Lilley JD. Role of anaerobic species in endodontic infection. Clin Infect Dis. 1997;25(2):220-1.
13. Egan MW, Spratt DA, Ng YL, Lam JM, Moles DR, Gulabivala K. Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int Endod J. 2002;35(4):321-9.
14. Fabricius L, Dahlén G, Holm SE, Möller AJR. Influence of combinations of oral bacteria on periapical tissue of monkeys. Scand J Dent. 1982;90(3):200-6.
15. Ferrari PHP, Cai S, Bombana AC. Effect of endodontic procedures on enterococci, enteric bacteria and yeasts in primary endodontic infections. Int Endod J. 2005;38(6):372-80.
16. Ferreira CM, Bonifácio KC, Fröner IC, Ito IY. Evaluation of the antimicrobial activity of three irrigating solutions in teeth with pulpal necrosis. Braz Dent J. 1999;10(1):15-21.
17. Gatti JJ, Dobeck JM, Smith C, White RR, Socransky SS, Skobe Z. Bacteria of asymptomatic periradicular endodontic lesions identified by DNA-DNA hybridization. Endod Dent Traumatol. 2000;16(5):197-204.
18. Gomes BP, Lilley JD, Drucker DB. Associations of endodontic symptoms and signs with particular combinations of specific bacteria. Int Endod J. 1996;29(2):69-75.
19. Gomes BPFA, Lilly JD, Drucker DB. Associations of specific bacteria with some endodontic signs and symptoms. Int Endod J. 1994;27(6):291-8.
20. Gomes BPFA, Pinheiro ET, Gade-Neto CR, Souza ELR, Ferraz CCR, Zaia AA et al. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19(2):71-6.
21. Georgopoulou M, Kontakiotis E, Nakou M. In vitro evaluation of the effectiveness of calcium hydroxide and paramonochlorophenol on anaerobic bacteria from the root canal. Endod Dent Traumatol. 1993;9(6):249-53.
22. Haapasalo M. Bacteroides spp in dental root canal infections. Endod Dent Traumatol. 1989;5(1):1-10.
23. Jacinto RC, Gomes BP, Ferraz CC, Zaia AA, Souza-Filho FJ. Microbiological analysis of infected root canals from symptomatic and asymptomatic teeth with periapical periodontitis and the antimicrobial susceptibility of some isolated anaerobic bacteria. Oral Microbiol Immunol. 2003;18(5):285-92.
24. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposure of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20(3):340-9.
25. Keudell K, Conte M, Fujimoto L, Ernest M, Berry HG. Microorganisms isolated from pulp chambers. J Endod. 1976;2(5):146-8.
26. Leonardi DP, Battisti JC, Klimiont DT, Tomazinho PH, Baratto-Filho F, Haragushiku GA et al. Avaliação in vitro da ação antimicrobiana de alguns cimentos endodônticos. RSBO. 2009;6(4):367-73.
27. Leonardo MR. Endodontia: tratamento de canais radiculares. Princípios técnicos e biológicos. 1. ed. São Paulo: Artes Médicas; 2005.
28. Möller AJ, Fabricius L, Dahlen G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89(6):475-84.
29. Morse DR. Endodontic microbiology in the 1970s. Int Endod J. 1981;14(2):69-79.
30. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. Manual of clinical microbiology. 6. ed. Washington: ASM Press; 1995.
31. Munson MA, Pitt-Ford T, Chong B, Weightman A, Wade WG. Molecular and cultural analysis of the microflora associated with endodontic infections. J Dent Res. 2002;81(11):761-6.
32. Peters LB, Wesselink PR, Buijs JF, Van Winkelhoff AJ. Viable bacteria in root dentinal tubules of teeth with apical periodontitis. J Endod. 2001;27(2):76-81.
33. Rôças IN, Siqueira Jr JF. Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse capture checkerboard approach. J Endod 2010;36(1):45-52.
34. Rocha MMNP, Moreira JLB, Menezes DB, Cunha MPSS, Carvalho CBM. Estudo bacteriológico de lesões periapicais. Rev Odontol Univ São Paulo. 1998;12(3):215-23.
35. Sakamoto M, Siqueira JF, Rôças IN, Benno Y. Diversity of spirochetes in endodontic infections. J Clin Microbiol. 2009;47(5):1352-7.
36. Shovelton DS. The presence and distribution of microorganisms within non-vital teeth. Br Dent J. 1964;117(35):101-7.
37. Siqueira Jr. JF, Rôças IN, Alves FRF, Campos LC. Periradicular status related to the quality of coronal restorations and root canal fillings in a Brazilian population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(3):369-74.
38. Siqueira Jr JF, Rôças IN. Community as the unit of pathogenicity: an emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:870-8.
39. Socranscky SS, Mcdonald JB, Sawyer S. The cultivation of Treponema microdentium as surface colonies. Arch Oral Biol. 1959;1(2):171-2.
40. Sundqvist G, Johansson E, Sjögren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989;15(1):13-9.
41. You YO, Kim KJ, Min BM, Chung EP. Staphylococcus lugdunensis – a potential pathogen in oral infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(3):297-302.
42. Winkler KC, Van Amerongen J. Bacteriologic results from 4.000 root canal cultures. Oral Surg Oral Med Oral Pathol. 1959;12(7):857-75.
43. Wittgow JR WC, Sabiston CB. Microorganisms from pulpal chambers of intact teeth with necrotic pulps. J Endod. 1975;1(5):168-71.
 Correspondence:
Correspondence:
Nadine Luísa Soares de Lima Guimarães
Rua Paulo Morais, n.º 860, apto. 904 – Papicu
CEP 60175-175 – Fortaleza – CE – Brasil
E-mail: nadine_guimaraes@hotmail.com
Received for publication: March 1, 2011
Accepted for publication: June 27, 2011













