Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.1 Joinville Jan./Mar. 2012
ORIGINAL RESEARCH ARTICLE
Human identification analysis using PCR from the root portion of dental elements under different conditions of temperature and exposure time
Ricardo Henrique Alves da Silva I Rodrigo Quiezi II Claudia Danielli Pereira Bertolaci II Suzana Papile Maciel Carvalho III Kellen Cristina da Silva Carina Thais de Almeida-e-Silva IV Lucilene Arilho Ribeiro Bicudo V
I School of Dentistry of Ribeirão Preto, University of São Paulo – Ribeirão Preto – SP – Brazil.
II Schoolof Genetics, University of São Paulo State – Botucatu – SP – Brazil.
III School of Dentistry, University of São Paulo – São Paulo – SP – Brazil.
IV School of Dentistry of Bauru, University of São Paulo – Bauru – SP – Brazil.
V University of São Paulo – Bauru – SP – Brazil.
ABSTRACT
Introduction and Objective: The main exogenous factors limiting the retrieval of information from human remains are fire and accidents involving high temperatures. Teeth, due to their relatively high degree of chemical and physical resistance, offer the possibility for the recovery of genetic material, becoming important in forensic cases. With the aim to contribute to a standardization of the protocols employed in DNA extraction and analysis, it was evaluated the integrity of DNA recovered from dental roots submitted to high temperatures, simulating what happens to burnt people. Material and methods: Extractions of genomic DNA were made from the dental root after exposure to high temperatures (600ºC, 800ºC and 1000ºC), during 10, 30 and 60 minutes. Results and Conclusion: After molecular analysis through PCR technique, it was verified that DNA amplification of the samples was not possible at any of the periods and temperatures analyzed.
Keywords: Forensic Dentistry; Forensic Anthropology; Forensic Sciences.
Introduction
By definition, identity is the set of characteristics that individualize a person or thing, making it distinct from all others. Identification is the act upon which identity of someone is established 2. França (2001) 10 considered that the value of postmortem identification is indisputable, considering either the social relations or the civil, administrative, commercial and penal requirements.
Forensic Dentistry has long contributed to these identification processes. Dentists, properly supported by Laws 6 and Resolutions 5 in Brazil, may perform analyses involving biological materials from the human body in various conditions (sliced, dilacerated, charred, macerated, decayed, in skeletonization and skeletonized), aiming to establish human identification 18.
Currently, with the application of molecular biology resources in human identification, it became possible to identify a person even without physical ante-mortem data or with deteriorated biological material in negligible amounts, which are actually relatively common conditions in forensic analyses 7,12,16,19. The new forensic sciences offer an unprecedented degree of certainty and reliability for the obtained results 19.
The main exogenous factors that may limit the retrieval of information from human remains and restrict the entire process of human identification are issues associated with fires, such as heat and explosions 23. In this context, teeth proved to be important elements in this type of identification, due to the high probability that there are not two people having the same exact dental features, as well as the relatively high degree of physical and chemical resistance of dental structures 15.
Teeth have in their composition the most resistant tissue in the human body – dental enamel – which provides high resistance against adverse conditions that can degrade DNA in the whole dental structure 14. Due to this resilience against environmental changes (incineration, immersion, traumas, mutilation and decomposition), teeth represent an excellent source of DNA, when the other biological sources have been lost 20,29,30. Also, due to the fact that dental pulp is surrounded by hard tissues, it is not easily influenced by changes in external temperature, as the buccal mucosa membrane and saliva 31.
PCR (polymerase chain reaction) technique has achieved increased importance for post-mortem DNA analysis in forensic cases 8 because of the millions of copies amplified from one specific sequence of DNA. Thus, it is of fundamental importance that dentists who act in Forensic Dentistry become familiar with the technological aspects of DNA analysis, as well as gradually incorporate this methodology into their practices 17.
It is not completely clear in which condition is possible to recover the DNA from teeth subjected to various temperatures under different exposure times, hence the expertise of Forensic Dentistry in human identification is necessary, especially in cases where little is left to make the identification, as in fires and explosions.
With the aim to evaluate the ability to recover genomic DNA contained in the root portion of teeth and its possible applicability in human identification process, human teeth were submitted to different heat conditions (600ºC, 800ºC, 1000ºC) for exposure times of 10, 30 and 60 minutes and the biological samples were amplified by PCR technique.
Material and methods
The project was submitted to the Committee for Ethics in Human Research of the Hospital for Rehabilitation of Craniofacial Anomalies at the University of São Paulo (CEP-HRAC-USP), approved under no. #117/2009-SVAPEPE-CEP.
Tooth collection
The teeth used in the study came from extractions of healthy maxillary and/or mandibular third molars, from Undergraduate and/or Graduate Dental Clinics at the School of Dentistry of Ribeirao Preto of the University of São Paulo (FORP-USP), upon receipt of a Patient Donation Letter and signing of an Informed Consent Form. The exclusion criteria consisted of the non-signing of the aforementioned documents or whether the extracted teeth were not healthy and intact.
Each group consisted of five healthy teeth, comprising a total of 45 teeth. Each person could donate one to four teeth, depending on the surgery. After the surgical procedure, the teeth were cleaned with sterile gauze and saline solution, placed in sterilized plastic recipients and stored in a freezer at -20°C until the moment of use.
Incineration procedure
Teeth were divided into groups, identified and subjected to incineration in a porcelain furnace (model EDGCON 3P-3000, Sao Carlos, SP, Brazil), using 10 ml porcelain crucibles, in the following conditions: group 1A (600ºC; 10 minutes); group 1B(600ºC; 30 minutes); group 1C (600ºC; 60 minutes); group 2A (800ºC; 10 minutes); group 2B (800ºC; 30 minutes); group 2C (800ºC; 60 minutes); group 3A (1000ºC; 10 minutes); group 3B (1000ºC; 30 minutes); group 3C (1000ºC; 60 minutes); group in natura (five teeth were not subjected to oven incineration).
DNA extraction from teeth
Teeth in natura were sliced side-to-side at the area of the cementum-enamel junction using a low rotation motor and a sterilized carborundum disc that was replaced for each tooth. For incinerated teeth, the crown was separated from the root, and the remaining roots were later pulverized, using sterilized #5 dental mirror handles that were replaced for each tooth. The removed tissues from all groups (experimental and control) were placed in a microcentrifuge tube, and genomic DNA was extracted according to the instructions of the Qiaamp DNA Micro Kit (Qiagen, Chicago, USA).
PCR technique application
To amplify the DNA from the samples, specific primers for the exon 2 of gene TGIF2 were employed. The sequence of this set of primers (Fw 5' CAATAGTTGCTGTGCTTATAAAGC 3', Rev 5' GAGTGGCAAGGAGCTTAATGC 3') produces a fragment with 378 bp. This human gene was chosen due to the countless other studies already performed with it at the Clinical Genetics Laboratory of the Hospital for Rehabilitation of Craniofacial Anomalies at USP (HRAC-USP). The protein codified by this gene is a DNA binding homeobox protein and a transcriptional repressor. This repressive role of transcription occurs by the recruitment of the histone deacetylase of genes responsive to TGFβ.
The amplification of the genomic DNA was executed in a 20 μl volume containing genomic DNA (60-100 ng), dNTPs (200 mM), primers (25 pmol each) and Taq polymerase (1U). The PCR Enhancer Kit (Invitrogen, San Diego, USA), consisting of 10x buffer, MgSO4 and Enhancer solution, was also used in all amplification reactions. The amplification conditions were 95ºC for 4 minutes, followed by 35 cycles at 95ºC for 30 seconds, 56ºC for 30 seconds, and 72ºC for 1 minute, and one extension at 72ºC.
After amplification, the products were separated by 1% agarose gel electrophoresis, stained with ethidium bromide. The products appear as a simple band corresponding to the size of the amplified sequence, which emits fluorescence when illuminated by ultraviolet light, as demonstrated in several works 1,3,24-26.
The samples were always handled using gloves and masks, in a sterile environment, and for very short times. The deionized water used in PCR reactions was autoclaved, as were the other solutions, except from the primers, dNTPs, buffer, and Taq DNA polymerase.
Results
Before performing the PCR assays, the DNA content was evaluated and values were estimated in ng/ml. For in natura group, values varied from 448 to 818, for group 1A from 188-300 and group 1B from 253-1188. For group 1C values were from 218 to 812 and 2A from 104 to 418. 2C and 3A values ranged from 78 to 293 and 40 to 372, respectively. Finally, for group 3B and 3C values varied from 45 to 85 and 62 to 88, respectively. This large range of the DNA content observed is probably a result of the morphological differences of the tooth and the individual variations.
The PCR reactions were prepared based on these values as an aim to optimize the PCR reactions. The PCR reactions were done for all samples, including experimental groups and positive and negative controls. As could be seen, the amplification was possible for teeth of in natura group (figure 1). On the other hand, all samples from those groups subjected to any kind of incineration did not allow the amplification, regardless of the periods or temperatures employed. The results indicated that amplification of the DNA was not possible in these samples using PCR (figures 2 and 3). An amplification of saliva samples was performed to show the applicability of the primer used in this study. All the samples collected from saliva shows amplification (figure 4).
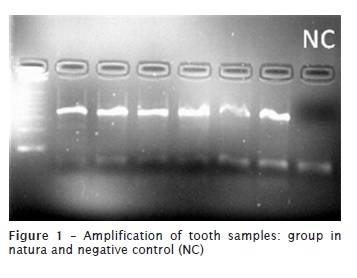
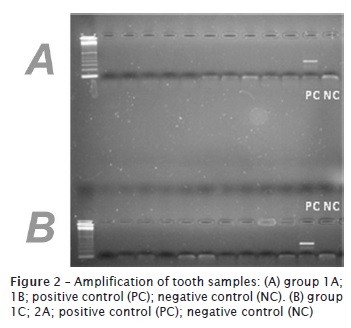
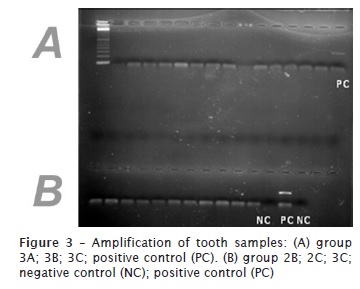

Discussion
Bohnert et al. (1998) 4 evaluated the changes seen during cremation in 15 bodies, verifying that facial bones were exposed only after 40 minutes when all soft tissue had been burnt and that the complete incineration had occurred after two to three hours. Based on this, times varying between 10 and 60 minutes were tested, once theoretically, the teeth would undergo direct exposure to fire for, at most, two hours. In another study, Rees (2010) 21 examined the effects of heat on the amplification of DNA from dental pulp of Sus scrofa molars and verified after incineration of intact heads for 15 min and 1h (average temperature of 625oC), amplifications of the largest fragment (450 bp) were successful from teeth. Moreover, the choice of temperatures was based on the fact that temperatures reached inside cremation ovens (670 to 800ºC) are similar to those reached in house fires, but with shorter duration due to firefighting actions 12,22. It is most similar to car fires, although temperatures can be higher, depending on the amount of fuel in the tank and the type of car 12.
Frequently, the biological materials found at the crime scene or collected from cadavers and mortal remains, present either on the victim's body or in objects, are scarce or degraded 9,22. Therefore, there is a concern about guaranteeing the preservation of the biological sample for DNA extraction and analysis, and these steps are specified in the few existing regulations, such as a Brazilian norm, Resolution SSP-SP no. #194 27, and a similar statute from FBI (2001) 9. In an attempt to mimic real identification cases, this study followed the regulations regarding collection and storage as recommended by these authorities. Thus, there was a great concern to avoid contamination by foreign DNA, which could hinder or mask the results obtained from the extraction of the sought DNA, especially because PCR technique makes possible the amplification of the DNA from a single cell, even if it is degraded 27. This single cell could have its DNA amplified along with the sought DNA, compromising the analysis of the genetic profile, and consequently making identification of the individual impossible. Furthermore, other cares were properly taken when performing PCR reactions to avoid contaminating the samples, such as physical isolation of the PCR preparation area and of the reagents employed, as well as control of the temperature, humidity and pH value, and correct choice of negative and positive controls, which can aid in detecting contaminants 9,22,27.
Bohnert et al. (1998) 4 conducted a study on the changes observed during the incineration of 15 bodies in temperatures ranging between 670ºC and 810ºC and verified that, after 60 minutes, teeth remained intact. Their study, however, was performed under controlled temperature conditions, in which the bodies were exposed in a uniform manner. This does not occur in fire accidents. In such cases, exposure is not always continuous, with temperature variations depending on the presence of flammable materials 14.
In accident cases, the tooth is protected by epithelial, conjunctive, muscle and bone tissue before being affected, as well as by the protection that the enamel, dentin and cementum provide to cells and tissues, thereby protecting the individual's genetic content. In this paper, the tooth was exposed directly to heat, without protection from those tissues. That direct exposure of the material to high temperatures of 600ºC, 800ºC and 1000ºC did not provide any favorable results to the amplification of the extracted DNA.
Although DNA concentration was favorable for later analyses, it was not possible to obtain any PCR product from the teeth submitted to heat. A first limiting factor is the fact that the integrity of the material was compromised. The exposure of the DNA molecule to high temperatures possibly led it to a fragmentation into smaller particles. This way, Schwark et al. (2010) 28, studying the identification of severely burnt human remains by genetic fingerprinting from bone fragments showed that the identification by DNA analysis is reliably and reproducibly possible from well preserved and semi-burnt bones.
Another important limiting factor for the amplification of these samples was the size of the primers used, 378 bp, which is a relatively large fragment for analysis of a degraded material. This indicates the relevance showing the size of the fragment to be amplified, as well as the integrity of the sample, which can contribute to the success of the analysis.
Another point to discuss about the results is the methodology employed to show the amplification. In this paper, an agarose gel was used because it is a low-cost and very easy procedure, but it is proved to be less sensitive than other gels. Although it was possible to see the amplification from the saliva samples, the experimental material, probably because of the degradation, could not be shown.
In a similar study, but with different temperatures, Tsuchimochi et al. (2002) 31 performed a procedure using Chelex 100 resin, in order to extract DNA from the dental pulp for later application of the PCR procedure. The extracted teeth were incinerated for 2 minutes at temperatures of 100ºC, 200ºC, 300ºC, 400ºC and 500ºC. All samples up to 300ºC were able to be amplified and classified, whereas no PCR product was obtained from those above 400ºC.
Malaver and Yunis (2003) 14 conducted research in order to evaluate different dental tissues as sources of DNA in forensic analysis, using 20 teeth obtained from unidentified bodies buried in 1995 and exhumed in 2000, obtaining 45 samples (5 of pulp, 20 of dentin, and 20 of cementum). The pulp produced the strongest signals of PCR amplification, while signals from dentin and cementum were quite similar to one another. This effect was expected once dentin and cementum are highly mineralized and present few organic content.
Hanaoka et al. (1995) 11 evaluated the extraction of DNA from 50 teeth (pulp and hard tissues). The DNA obtained from the dental pulp varied between 3 and 40 μg, and no correlation was found between the storage period of samples and the amount of DNA found. The authors investigated the efficiency of DNA extraction from hard dental tissues in different concentrations of decalcification solution. The DNA obtained from dental pulp had high molecular weight, and was susceptible to analysis by multi-locus probe or PCR; the material obtained from hard tissues showed a satisfactory analysis only with PCR.
In a similar study, Remualdo et al. (2004) 22 evaluated the PCR amplification of DNA obtained from teeth submitted to heat (200ºC, 400ºC, 500ºC and 600ºC) during 60 minutes, testing three extraction methods (organic; ammonium isopropyl/acetate; and silica). For PCR amplification of DNA, primers of genomic DNA (STR-F13A01) and mitochondrial DNA (MPSs) were employed. Using the organic method to extract genomic DNA, 50% of samples subjected to incineration were amplified, although at lower temperatures (200ºC and 400ºC). At higher temperatures (500ºC and 600ºC), the ammonium isopropyl/acetate method produced the best results for the extraction of mitochondrial DNA.
Conclusion
Using a commercial kit for DNA extraction and amplification of the human gene TGIF2 in the samples, the present study verified that it was not possible to recover genomic DNA contained in the root portion of the teeth for human identification purposes when submitted to different heat and exposure time conditions. It is advisable that new assays are performed using a primer with smaller size for more conclusive results.
Acknowledgements
We thank to the Molecular Biology Laboratory at HRAC-USP for cooperating with this study; to Prof. César Antunes de Freitas (PhD, Dental Materials) for the access given to equipment for incineration of teeth; and to FORP-USP professors: Maria José Alves da Rocha, Raquel Fernanda Gerlach and José Moacir Marin, for providing materials for the study.
References
1. Akane A, Seki S, Shiono H, Nakamura H, Hasegawa M, Kagawa M et al. Sex determination of forensic samples by dual PCR amplification of an X-Y homologous gene. Forensic Sci Int. 1992;52(2):143-8. [ Links ]
2. Almeida-Junior A, Costa-Junior JBO. Lições de medicina legal. São Paulo: Companhia Editora Nacional; 1998.
3. Becari C, Sivieri DO, Santos CF, Moysés MK, Oliveira EB, Salgado MC. Role of elastase-2 as an angiotensin II-forming enzyme in rat carotid artery. J Cardiovasc Pharmacol. 2005;46(4):498-504.
4. Bohnert M, Rost T, Pollak S. The degree of destruction of human bodies in relation to the duration of the fire. Forensic Sci Int. 1998;95:11-21.
5. Brasil. Conselho Federal de Odontologia. Resolução CFO 22/2001. Baixa normas sobre anúncio e exercício das especialidades odontológicas e sobre cursos de especialização, revogando as redações do Capítulo VIII, Título I; Capítulos I, II e III, Título III, das normas aprovadas pela Resolução CFO-185/93, alterada pela Resolução CFO-198/95. 27 dez. 2001 [cited 2009 Nov 4]. Available from: URL: http://cfo.org.br/servicos-e-consultas/ato-normativo/?id=378.
6. Brasil. Lei n.º 5.081, de 24 de agosto de 1966. Regulamenta o exercício da Odontologia no Brasil. Diário Oficial da União, Brasília (DF); 1966.
7. Corach D, Sala A, Penacino G, Iannucci N, Bernardi P, Doretti M et al. Additional approaches to DNA typing skeletal remains: the search for "missing" persons killed during the last dictorship. Electrophoresis. 1997;18(9):1608-12.
8. Farah SB. DNA e a lei. In: Farah SB. DNA: segredos e mistérios. São Paulo: Sarvier; 1997.
9. FBI. Federal Bureau of Investigation. Quality assurance audit for forensis DNA and convicted offender DNA databasing laboratories. 2001. Available from: URL: http://home.att.net/~applieddnaresources/DNA_Audit.pdf.
10. França GV. Medicina legal. Rio de Janeiro: Guanabara Koogan; 2001.
11. Hanaoka Y, Inoue M, Tsai TH, Minaguchi K. Fundamental and practical study for DNA analysis using tooth as a source of DNA. Japanese J Legal Med. 1995;49(1):1-10.
12. Houck MM. CSI: reality. Scientific American. 2006;295(1):84-9.
13. Kalmár T, Bachrati CZ, Marcsik A, Raskó I. A simple and efficient method for PCR amplifiable DNA extraction from ancient bones. Nucleic Acids Res. 2000;28(12):e67.
14. Malaver PC, Yunis JJ. Different dental tissues as source of DNA for human identification in forensic cases. Croat Med J. 2003;44(3):306-9.
15. Miyajima F, Daruge E, Daruge-Júnior E. A importância da odontologia na identificação humana: relato de um caso pericial. Arq Odontol. 2001;37(2):133-42.
16. Murphy E. The new forensics: criminal justice, false certainty, and the second generation of scientific evidence. Available from: URL: http://ssrn.com/abstract=896128.
17. Ng DP, Koh D, Choo SG, Ng V, Fu Q. Effect of storage conditions on the extraction of PCR-quality genomic DNA from saliva. Clin Chimica Acta. 2004;343:191-4.
18. Oliveira RN, Daruge E, Galvão LCC, Tumang AJ. Contribuição da odontologia legal para a identificação post-mortem. Rev Bras Odontol. 1998;55(2):117-22.
19. Prainsack B, Kitzberger M. DNA behind bars: other ways of knowing forensic DNA technologies. Social Studies Science. 2009;39(1):51-79.
20. Pretty IA, Sweet D. A look at forensic dentistry – part 1: the role of teeth in the determination of human identity. Brit Dent J. 2001;190(7):359-66.
21. Rees KA, Cox MJ. Comparative analysis of the effects of heat on the PCR-amplification of various sized DNA fragments extracted from Sus Scrofa molars. J Forensic Sci. 2010;55(2):410-7.
22. Remualdo V, Nunes FD, Hirata M, Melani RFH, Oliveira RN. Análise do STR-F13A01 e MPSs do mtDNA para fins de identificação humana: comparação de três métodos de extração de DNA de dentes submetidos ao calor. RPG Rev Pós-Grad. 2005;12(4):437-43.
23. Rodrigues RJ, Melani RFH. Identificação humana em vítimas de carbonização: análise odontolegal através da microscopia eletrônica. Rev Pós-Grad Fac Odontol Univ São Paulo. 2001;8(3):261.
24. Santos CF, Caprio MA, Oliveira EB, Salgado MC, Schippers DN, Munzenmaier DH et al. Functional role, cellular source, and tissue distribution of rat elastase-2, an angiotensin II-forming enzyme. Am J Physiol Heart Circ Physiol. 2003;285(2):H775-83.
25. Santos CF, Oliveira EB, Salgado MC, Greene AS. Molecular cloning and sequencing of the cDNA for rat mesenteric bed elastase-2, an angiotensin II-forming enzyme. J Cardiovasc Pharmacol. 2002;39(5):628-35.
26. Santos CF, Sakai VT, Machado MAAM, Schippers DN, Greene AS. Reverse transcription and polymerase chain reaction: principles and applications in dentistry. J Appl Oral Sci. 2004;12(1):1-11.
27. São Paulo. Secretaria de Segurança Pública. Resolução SSP-194, 2 de junho de 1999. Diário Oficial do Estado, 1999 Jun. 3; seção 1:104.
28. Schwark T, Heinrich A, Preuße-Prange A, Von Wurmb-Schwark N. Reliable genetic identification of burnt human remains. Forensic Sci Int Genet. 2011 Nov;5(5):393-9.
29. Silva RHA, Musse JO, Melani RFH, Oliveira RN. Human bite mark identification and DNA technology in forensic dentistry. Braz J Oral Sci. 2006;5:1193-7.
30. Silva RHA, Sales-Peres A, Oliveira RN, Oliveira FT, Sales-Peres SHC. Use of DNA technology in forensic dentistry. J Appl Oral Sci. 2007;15(3):156-61.
31. Tsuchimochi T, Iwasa M, Maeno Y, Koyama H, Inoue H, Isobe I et al. Chelating resin-based extraction of DNA from dental pulp and sex determination from incinerated teeth with y-chromosomal alphoid repeat and short tandem repeats. Am J Forensic Med Pathol. 2002;23(3):268-71.
 Correspondence:
Correspondence:
Ricardo Henrique Alves da Silva
Faculdade de Odontologia de Ribeirão Preto – Universidade de São Paulo
Departamento de Clínica Infantil e Odontologia Preventiva e Social
Área de Odontologia Legal
Avenida do Café, s/ n.º – Monte Alegre
CEP 14040-904 – Ribeirão Preto – SP – Brasil
E-mail: ricardohenrique@usp.br
Received for publication: May 13, 2011
Accepted for publication: September 12, 2011













