Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.2 Joinville Abr./Jun. 2012
Original Research Article
Interference of the assessment method in pH values of an epoxy-based cement
Daniele Fockink da Silva I; Elize Cristina Stang I; Edson Alves de Campos II; Milton Carlos Kuga II; Gisele Faria II; Gabriel Keine Kuga III
I School of Dentistry, FUNEC – Santa Fé do Sul – SP – Brazil.
II School of Dentistry of Araraquara, Universidade Estadual Paulista – Araraquara – SP – Brazil.
III School of Sciences and Technology, Universidade Estadual Paulista – Presidente Prudente – SP – Brazil.
ABSTRACT
Introduction :Alkalinization potential is a fundamental property of endodontic epoxy-based cements containing calcium hydroxide. Studies have shown discrepant pH results for same materials at different evaluation periods. A possible reason accounting for these differences may be the assessment procedures. Objective: To evaluate the pH value of an epoxy-based cement (Sealer 26) in different periods of analysis, using two assessment methods. Material and methods:Sealer 26 was manipulated and immediately placed into polyethylene tubes (n=10, each group) and immersed in distilled water. In G1, the tubes were kept in the same water during all experiment; and in G2, the tubes were removed and placed into another flask with an equal amount of water after the pH evaluation. The pH of these solutions was measured at 24 hours, 7, 14 and 28 days. Analysis were made within the same group according to the experimental periods and between groups in each experimental period. Data were submitted to ANOVA (a = 5%) and t test, respectively.Results:For G1 and G2, all periods showed different pH values (p < 0.05), except between 14 and 28 days (p > 0.05) and between 7 and 14 days (p > 0.05), respectively. In each period, no significant differences were observed between the groups.Conclusion: The method to obtain the pH values in different experimental periods no interfered in the final results. However, difference was observed when the results were analyzed at same group
Keywords: pH; resin cement; distilled water.
Introduction
Endodontic cements should have both antimicrobial action 15 and the capacity to stimulate the healing of periapical tissue 9. Antimicrobial action is related to the release of hydroxyl ions 4 increasing pH and creating an unsuitable environment for microbial growth 6.
The pH of a solution or substance is usually measured with a specific device (pHmeter) using the electrode directly into the substances 13 or indirectly in the solutions in which the specimens were immersed 8. Sometimes, in the analysis with the same material, in similar conditions of manipulation and at the same period of analysis, different values are obtained, as observed in studies with MTA-based cements 5,14. Possible reasons to explain these results could be either different procedures to analyze the pH changing or the distilled water in which the specimens remained immersed, but no study has confirmed these hypotheses.
Sealer 26 cement is a root canal filling material commonly used in Brazil. According to the manufacturer, the main constituents of Sealer 26 are: a) powder (bismuth trioxide, calcium hydroxide, hexametilene tetramine, titanium dioxide) and b) resin (Bisphenol-epoxy resin). Silva et al. (1997) 11 observed that the highest pH values were obtained for Sealapex in comparison with CRCS, Apexit and Sealer 26. Duarte et al. (2000) 3 obtained similar results, except that Sealer 26 showed highest pH during the initial periods. However, after its final setting, Sealer 26 pH decreased its pH 2.
Santos et al. (2005) 10 recommended the specimens change into another flask with an equal amount of distilled water to avoid saturation of the medium. On the other hand, Gonçalves et al. 5 assessed the pH without liquid substitution, because other reactions may occur, such as the formation of different substances involving hydroxyl ions ionized from Ca(OH)2 and interfere with the real pH values of the cement 11.
The aim of this study was to evaluate the interference of the method for obtaining the pH values of the epoxy-based cement (Sealer 26), at 24 h, 7, 14, and 28 days, within the same group and between each experimental period. The null hypothesis is that the method influences pH value assessment, within the same group throughout the experiment and between the groups at each evaluation period.
Material and methods
Throughout the experiment, Sealer 26 (Dentsply Ind Com Ltda, Rio de Janeiro, RJ, Brazil) was prepared according to the manufacturers ratio recommendation: 2.0 g powder: 1.0 g resin 3. Twenty polyethylene tubes measuring 0.5 cm length and 1.0 mm internal diameter were filled with Sealer 26 (Dentsply) using a Lentulo spiral (Maillefer, Ballaigues, Switzerland). The tubes filled with fresh cement were weighed in order to check the standardization of the amount of cement.
In sequence, the specimens were divided into two groups (n = 10), according to pH assessment method: (G1) the tubes were kept into the same distilled water throughout the experiment; (G2) the tubes were removed and placed into another flask with an equal amount of distilled water after the pH evaluation.
They were placed into polypropylene closed flasks (Injeplast, São Paulo, SP, Brazil) containing 10 mL of distilled water and kept in oven at 37°C (Farmen, São Paulo, SP, Brazil). Prior to the specimens immersion, the distilled water showed a pH of 6.8. At 3 hours, all specimens of G2 were placed into another flask with the same volume of new distilled water. The specimens of G1 were kept into the same distilled water. Measurement of pH was performed with a pHmeter (model DM22, Digimed, São Paulo, SP, Brazil), previously calibrated with solutions of known pH (4, 7 and 14), at constant room temperature (25°C), after 24 hours, 7, 14 and 28 days. After removal of the specimens, the flasks were placed in a shaker (251, Farmen) for 5 seconds before pH measurement. Control sample comprised pH measuring of the water in which no specimens had been immersed. Each group data at the several periods were analyzed by one-way ANOVA (a = 5%). t test (a = 5%) was used to compare between groups at each period
Results
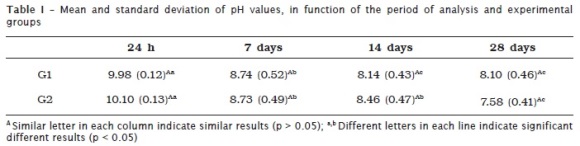
Table I shows the pH values provided by the tested groups (G1 and G2) at 24 hours, 7, 14 and 28 days of immersion. In both groups, there was a decreased of pH value during the experiment. In G1, pH value was different at all periods, except between 14 and 28 days. In G2, pH value was different at all the periods, except between 7 and 14 days. There was no difference at each experimental period between the groups (table I).
Discussion
The method to assess the pH values of Sealer 26 cement no interfered in the final results. When the specimens are kept into the same distilled water there was a tendency to stabilize the pH after 14 days. On the other hand, when the specimens are placed into new distilled water the differences tended to occur regardless of the period of immersion. However when the groups were compared at each period, no statistical differences were found. Thus, the null hypothesis is rejected. The maintenance of the specimens in the same water should be performed when one aims to establish the balance of pH values. When the aim is to assess the pH value at an each specific time period, it is recommended to place the specimens into new amount of distilled water.
Several methods to assess the pH of endodontic cement have been proposed 2,3,8,14. Notwithstanding, the results interpretation of the different methods are controversial and conflicting. Small modifications at the moment of obtaining the pH value may lead to variable results. When the specimen is kept into the same solution during all experiment, at a determinate moment, there is a tendency toward balance because of the saturation process of the solution 11. This method enables to check whether other reactions may occur, such as the formation of different substances involving hydroxyl ions ionized by the calcium hydroxide-containing cement (Sealer 26) 11, mainly during the setting time of the cement 2, which directly interferes in the pH values 3,11.
The advantage of placing the specimens into other flasks during the experiment is the possibility of quantifying the hydrogen ion released at each period 10 and to prevent the distilled water saturation 10. This method was used in several studies 3,7,8,10,12. In our study, after 3 hours of the beginning of the experiment, the specimens were removed from the initial flasks and placed into new distilled water. This procedure was intended to differentiate G2 from G1 group, at 24 hours period.
This study used as reference the epoxy-based cement (Sealer 26). Sealer 26 (Dentsply) contains calcium hydroxide in composition (0.370 g/g cement), and demonstrates favorable biological behavior 1. The presence of some acid-hydrogen components in the cement, as bisphenol-epoxy, may have reacted with hydroxyl ion causing a pH decrease 11, which was mainly detected in G1 group after 14 days.
The results obtained in this present study cannot be fully transferred to clinical application because clinical conditions may interfere in the results. Further clinical and laboratorial studies should be carried out regarding to pH values of the Sealer 26 cement.
Conclusion
pH assessment procedures of the materials no influenced in the final results. The method of specimens changing into new distilled water provides more realistically values of hydrogen ion release, at each analysis period; on the other hand, the specimens maintenance into the same distilled water allows visualization of pH values behavior throughout the experiment.
References
1. Borlina SC, Souza V, Holland R, Murata SS, Gomes-Filho JE, Dezan-Junior E et al. Influence of apical foramen widening and sealer on the healing of chronic periapical lesions induced in dogs’ teeth. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2010 Jun;109(6):932-40.
2. Carneiro DF, Barbosa SV. Avaliação do pH dos cimentos endodônticos e considerações clínicas. ROBRAC. Jan-Jun;7(24):6-10.
3. Duarte MAH, Demarchi ACCO, Giaxa MH, Kuga MC, Fraga SC, Souza LCD. Evaluation of pH and calcium ion release of three root canal sealers. J Endod. 2000 Jul;26(7):389-90.
4. Estrela C, Pesce HF. Chemical analysis of the liberation of calcium hydroxyl ions from calcium hydroxide pastes in connective tissue in the dog. Part I. Braz Dent J. 1996 Jan-Jun;7(1):41-6.
5. Gonçalves JL, Viapiana R, Miranda CES, Borges AH, Cruz-Filho AM. Evaluation of physico-chemical properties of Portland cements and MTA. Braz Oral Res. 2010 Jul-Sep;24(3):277-83.
6. Haapasalo M, Ostarvik D. In vitro infection and desinfection of dentinal tubules. J Dent Res. 1987 Aug;66(8):1375-9.
7. Kuga MC, Campos EA, Sant’Anna Junior A, Vasconcelos FL, Silva AN, Nascimento CA. Avaliação do pH, da solubilidade e da infiltração marginal em obturações com o Sealer 26® puro ou acrescido de iodofórmio. RSBO. 2010 Oct-Dec;7(4):389-95.
8. Kuga MC, Campos EA, Viscardi PH, Carrilho PZ, Xavier FC, Silvestre NP. Hydrogen ion and calcium releasing of MTA Fillapex® and MTA-based formulations. RSBO. 2011 Jul-Sep;8(3):271-6.
9. Mutoh N, Tani-Ishii N. A biocompatible model for evaluation of the responses of rat periapical tissue to a new zinc oxide-eugenol sealer. Dent Mater J. 2011 Mar;30(2):176-82.
10. Santos AD, Moraes JCS, Araújo EB, Yukimitu K, Valério-Filho WV. Physico-chemical properties of MTA and a novel experimental cement. Int Endod J. 2005 Jul;38(7):443-7.
11. Silva LAB, Leonardo MR, Silva RS, Assed S, Guimarães LEL. Calcium hydroxide root canal sealers: evaluation of pH, calcium ion concentration and conductivity. Int Endod J. 1997 May;30(3):205-9.
12. Vasconcelos BC, Bernardes RA, Cruz SML, Duarte MA, Padilha PM, Bernardineli N et al. Evaluation of pH and calcium ion release of new root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Jul;108(1):135-9.
13. Vianna ME, Zilio DM, Ferraz CCR, Zaia AA, Souza-Filho FJ, Gomes BPFA. Concentration of hydrogen ions in several calcium hydroxide pastes over different periods of time. Braz Dent J. 2009 Dec;20(5):382-8.
14. Vivan RR, Zapata RO, Zeferino MA, Bramante CM, Bernadinelli N, Garcia RB et al. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2010 Feb;110(2):250-6.
15. Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009 Jul;35(7):1051-5.
 Correspondence:
Correspondence:
Milton Carlos Kuga
Av. Saul Silveira, 5-01
CEP 17.018-260 – Bauru – SP – Brasil
E-mail:kuga@foar.unesp.br
Received for publication: October 27, 2011.
Accepted for publication: December 14 2011.













