Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.3 Joinville Jul./Set. 2012
Original Research Article
Fluoride effect on the process of alveolar bone repair in rats: evaluation of activity of MMP-2 and 9
Mileni da Silva Fernandes I,II; Gisele Miyamura Martins III ;Lucas Makoto Shimoraha II ;Flávia Godoy Iano II ; Marcela Mitsuko Yanai IV ; Tânia Mary Cestari II ; Marília Afonso Rabelo Buzalaf II; Rodrigo Cardoso de Oliveira II
II School of Dentistry of Bauru, University of São Paulo – Bauru – SP – Brazil.
III Institute of Biomedical Sciences, University of São Paulo – São Paulo – SP – Brazil.
IV Private practice.
ABSTRACT
Objective: The aim of this study was to evaluate comparatively the effect of fluoride (F) on the activity of matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) involved in process of alveolar bone repair. Material and methods: This study used 4 groups of Wistar rats with 80 days of life (n = 160) which received drinking water containing different doses of fluoride (NaF): 5, 15, 50 ppm and deionized water (control) throughout the experiment. These animals had their right upper incisors extracted. After extraction, the animals were euthanized at 7, 14, 21 and 30 days and the hemi-maxillae were collected for microscopic analysis (Hematoxylin and Eosin and immunohistochemistry for MMP-9) and zymography (MMP-2 and 9).Results: Microscopically the process of bone repair was similar in all groups, being noted only a delay of the blood clot resorption and bone formation in the group of 50 ppm F. The expression for MMP-9 showed differences betweengroups only during the initial repair (7 days). However, the zymography showed no significant differences between treated and control groups. Conclusion: Ours results suggest an effect of fluoride on the activity of MMPs 2 and 9 at the initial period of alveolar repair which could be associated to the process of blood clot remission and delay in bone repair. Further studies are needed to establish the relationship between the initial process of resorption of the blood clot, and the involvement of MMPs 2 and 9 and its regulators/tissue inhibitors.
Keywords: bone repair; matrix metalloproteinases; fluoride.
Introduction
The process of bone repair is an event finely regulated and characterized by different phases with predominance of specific cellular types, aiming at the formation of tissue in the affected area. The repair stages involve migration, proliferation, differentiation, and activation of numerous cellular types 16,22. Among the different study models of bone repair, the model of bone repair in tooth socket has been very adequate because it enables a chronological evaluation of the repair process, detailing the main cellular events at each stage 31.
Chronologically, four fundamental stages can be considered in the evolution of the alveolar repair process 30,37: 1) exudative stage, characterized by the filling of the tooth socket by blood clot (1-7 days after tooth extraction, in alveolus of rats); 2) proliferative stage, marked by intense cellular proliferation, development and maturation of connective tissue (7-14 days after tooth extraction, in alveolus of rats); 3) reparative stage, in which occurs the gradual reposition of the connective tissue by bone trabeculae (14-21 days after tooth extraction, in alveolus of rats); 4) remodeling stage, characterized by the substitution process of replacement of primary by secondary bone tissue (21 days after tooth extraction, in alveolus of rats) 1.
Considering from the initial stage of blood clot formation to the last stage of newly-formed bone tissue remodeling, several cells and signaling molecules are involved which regulate (and are also regulated) during the development of this process. Some molecules are involved in several stages of the process of alveolar repair, for example matrix metalloproteinases (MMPs) 2, among others. MMPs are an important family of metal-dependent endopeptidases and represent the major class of enzymes responsible for the degradation or resorption of all components of the extra-cellular matrix (ECM) 38. Their targets include other proteinases, proteinase inhibitors, blood clot factors, chemotactic molecules, latent growth factors, growth factor-binding protein, receptors of cell surface, cell adhesion molecules 29. MMPs can be classified according to their structure or their specific substrates, resulting in 25 types of MMPs 15.
Many of the crucial responses at the repair stages, such as inflammatory infiltrate, angiogenesis (degradation of the basal membrane, invasion, proliferation and capillary formation) 15, and re-epithelization are modulated by MMPs; also these molecules may be important markers of tissue repair, especially MMPs known as gelatinases (MMP-2 e MMP-9) 39. They are involved in the alveolar bone repair of rats 1, osteogenesis 13, and they participate in the last stages of ossification 41.
Within the current knowledge on the biology of bone repair, it is known that fluoride (F) has been used as an alternative for stimulating the mitosis of osteoblasts, and it has been researched as an anabolic agent in bone tissue for the treatment of osteoporosis 35. Several studies have demonstrated the effect of fluoride in osteoblasts 4,8,9,23,31 and molecules as actin, enzymes (alkaline phosphatase) 32 etc. However, details about the effects of fluoride on some molecules involved in bone repair process, among them MMPs, have not been fully understood yet 19. Since fluoride has been used as public health measure in Brazil (within the water supply), it is worth investigating some of these possible effects on bone tissue repair, specifically regarding to MMPs. Therefore, this study aim to evaluate the effect of fluoride on the activity of MMPs 2 and 9 involved in the process of alveolar bone repair.
Material and methods
Animals
The study was previously approved by the Ethical Committee in Teaching and Research in animals of the School of Dentistry of Bauru – University of São Paulo. Male Wistar rates (Rattus norvegicus), coming from the Central Vivarium of the School of Dentistry of Bauru, were used. The animals (n = 160) were divided into 4 experimental groups (0 ppm, 5 ppm, 15 ppm and 50 ppm F) and treated with the controlled ingestion of fluoride (drinking water and food) for 60 days just after ablactation. The water was prepared with different F concentrations according to the aforementioned groups: 0 ppm (deionized water); 5 ppm (water with 5 ppm of fluoride); 15 ppm (water with 15 ppm of fluoride); 50 ppm (water with 50 ppm of fluoride).
During all the study (pre- and post-surgery), the food contained low fluoride content 33.
Surgical procedures
After the treatment period (60 days), the animals were submitted to the extraction of the upper right incisor, performed under general anesthesia by ketamine and xylazine (1:1), calculated according to the body mass of the animal (0.14 ml/100 g of weight), by intramuscular via 26. Suture was accomplished through black silk thread (4/0 Ethicon, Johnson & Johnson, São José dos Campos, SP, Brazil), according to the procedures previously described by Okamoto and Russo 26.
Collection and histotechnical processing of the samples for microscopy
Elapsed the experimental periods, the animals were euthanized according to the guidelines of the Brazilian College of Animal Experimentation (short Cobea) by excessive dosage of anesthetic drug, and the right maxilla was collected. Half of the pieces collected were destined to histotechnical processing and the other half to zymography. For histological and immune-histochemical analysis, the pieces obtained (n = 5) were submitted to fixation in 10% buffered formalin, for 48 hours; next, they were radiographed, demineralized in 0.05 M ethylenediamine tetraacetic acid (EDTA) solution, pH 7,4, 27, and diaphanized for inclusion in paraffin. Following, 4 µm-thick cuts were performed in microtome (Microm, model HM 340 E, Germany), included in conventional and Super-frost Plus silanized laminas (Erviegas, São Paulo, SP, Brazil), which were respectively stained by hematoxylin-eosin (HE) technique and immunostained against specific antigens.
Microscopic analysis of the laminas stained by HE
The laminas stained by hematoxylin -eosin technique were examined under light microscopy. The tooth socket was evaluated in its apical and medium thirds. The biological response was analyzed according to the stages of the repair process: presence of the blood clot, fibroblastic proliferation and bone neoformation.
Immunohistochemistry
To detect MMP-9 protein, the cuts were submitted to immunostaining technique through indirect via by SABC peroxidase method. Three laminas per animal were prepared and in each one ten fields of 6,300 mm2 were marked to determine the counting of the immunostained cells for the specific antibody. The tissue cuts were diaphanized (3 baths of 5 minutes in xylol) and rehydrated in decreasing ethanol concentrations (100%, 95% and 70%) for 5 minutes each and subsequently in distilled water. The inactivation of the endogenous peroxidases was performed in 3% hydrogen peroxide (2 baths of de 10 minutes). Next, it was executed PBS washing (2x), exposition of antigens by pepsin enzymatic digestion (S3002, Dakocytomation Carpinteria, USA) for 20 minutes, washing in 3 baths of PBS (3x), blockage of the serum proteins performed in a 10% solution of skim milk (Molico, Nestlé Brazil Ltd., Araçatuba, São Paulo, Brazil) in PBS for 40 minutes. The laminas were incubated with primary antibody against MMP-9 (SC6840, Santa Cruz Biotechnology Inc, USA; 1:100) diluted in antibody diluent (S3022 Dakocytomation Carpinteira, USA) for 1 hour and 30 minutes at environmental temperature. The negative control cuts were incubated in PBS solution. After, all tissue cuts were washed in PBS (3x). The incubation with the secondary antibody (E0466 – Dakocytomation Carpinteira, EUA; 1:500) was performed for 1 hour. After this period the cuts were submitted to 3 baths of PBS. The detection of the primary-secondary antibody complex was accomplished by the incubation in estreptavidine-HRP (StrptABComplex/HRP®, LSAB2 –X0909 or K0675 – DakoCytomation Carpinteria, USA) for 30 minutes. Following, PBS baths (3x), visualization of the antigen-antibody reaction with DAB+ (K3468-DakoCytomation Carpinteria, USA) for 45 s, followed by PBS and distilled water washing (5 min, 3x) were executed. Finally, counterstaining by hematoxylin for 45 s, water washing for 10 min, ethanol dehydration, xylol diaphanization and adhesion of the coverslip in the cuts with Enterlan® resin (Merck KGaA, Frankfurter, Darmstadt, Germany) were performed.
The cells immunostained for MMP-9 protein were analyzed in x100 magnification objective, with reticular integration, coupled to x8 magnification ocular. The results were obtained in number of cells/mm2.
Zymography analysis
The samples collected for the zymographic analysis (n = 5) were triturated and homogenized at low temperature (-170°C) by using a cryogenic grinding mill (6770 Freezer/Mill, Spex Certiprep, Metuchen, NJ, USA). To extract the proteins, approximately 1 g of alveolar bone was homogenized in triton X100 at 25% and agitated. The samples were centrifuged for 20 min at 15,000 rpm, at +4°C. At the end, the supernatant was discard and 200 µL of buffer extraction Tris 50 mM and CaCl2 100 mM, pH 7.4 and 1 µL of PMSF (phenylmethylsulfonyl fluoride) was added to the precipitate. The samples were left in water bath at 50°C for 2 hours, and at each 10 minutes a light agitation was performed; after that, the samples were centrifuged for 15 minutes at 15,000 rpm. The supernatant was collected and kept in freezer (-20°C) for following quantification by using the method described by Lowry et al. 21.
In electrophoresis, it was applied 60 µg of protein from the tooth socket samples. It was used a separation gel at 11% sodium dodecyl sulphate polyacrylamide (SDS-PAGE) with 0.5% gelatin as substrate. Molecular weight patterns were used for MMP-2 and 9 (Calbiochem, EMD, Biosciences Inc., La Jolla, CA, USA).
At the electrophoresis end (approximately 2 hours) the gel was incubated in 50mM Buffer Tris HCl, 2.5% Twenn 80, 0.02% NaN3, pH 7.5, and left for 30 minutes; next it was replaced by 50 mM Buffer Tris HCl, 2.5% Twenn 80, 0.02% NaN3, 1 µm ZnCl2, 5 mM CaCl2 and left for more 30 minutes. Then, it was replaced for 50mM Buffer Tris HCl, 5mM CaCl2, 0.02% NaN3, 1 µm ZnCl2 (pH 7.5), in which the gel was left for 18 hours at 37°C. At the end of this period, the gel was stained by Coomassie Blue G-250 (0.5%) and bleached by a solution of 10% methanol and 5% acetic acid. The gel analyses were performed by densitometry through Kodak Molecular Imaging Software (Rochester, NY, USA).
Statistical analysis
GraphPad Instat software version 3.0 for Windows and GraphPad Prism software version 4.0 for Windows (Graph Pad Software, San Diego, USA) was used. Data presented a normal and homogenous distribution, and then they were analyzed by Anova. Tukey test was applied as post hoc for Anova. The level of significance was adopted at 5%, for all cases.
Results
Microscopic analysis
In all groups studied, the process of repair was noted according to the time elapsed during the experimental periods (7, 14, 21 and 30 days). At 7 days, the tooth socket was occupied mainly by blood clot and connective tissue, without great differences among groups. At 14 and 21 days, there was a gradual decreasing of the blood clot, an alteration in the connective tissue composed by fibroblasts, macrophages, and some inflammatory cells to conjunctive tissue composed by fibroblasts and fibrocytes, parallely to the substitution by newly bone tissue (primary), growing from the socket walls to its center. At these time periods, (14 and 21 days), a greater bone tissue formation was noted in the control group in comparison with the groups treated with fluoride; also, there was a higher presence of blood clot vestiges in the group treated with 50 ppm F. At 30 days, the formation of bone tissue covering great part of the socket was seen in all groups; however, there was a higher bone formation in control group compared with the group treated 50 ppm F.
MMP-9: immunostaining and histomorphometry
In general, the cells immunostained for MMP-9 were mononucleated cells similar to macrophages, at the initial periods (7 and 14 days), found in all groups. At the following periods, 21 and 30 days, the staining was predominantly in osteoblasts, mononucleated cells similar to fibroblasts and osteoclasts (figure 1), found in all groups studied.
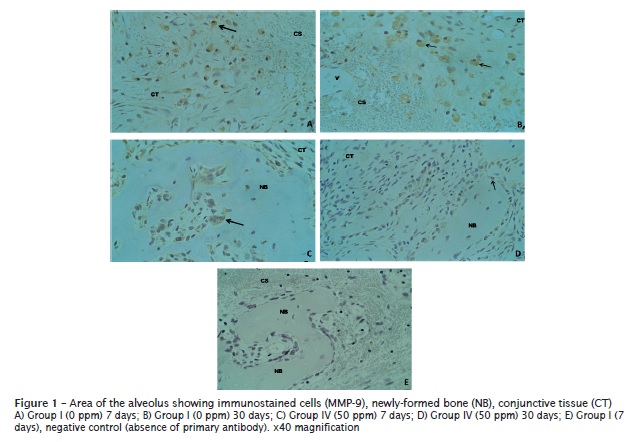
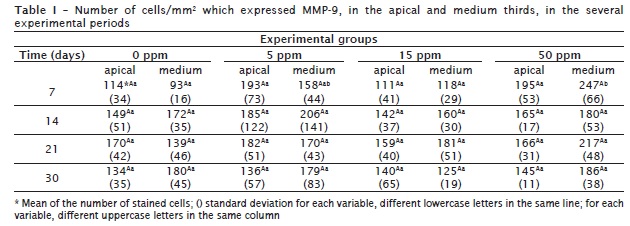
Apical third: The expression of MMP-9 was registered in all groups, both experimental and control, (figure 1). The expression of MMP-9 increased in 0 ppm and 15 ppm groups, according to the period of time elapsed (7-30 days). However, in 5 ppm and 50 ppm F groups there was a decreasing in the number of cells immunostained for MMP-9. There were no statistically significant differences in the expression of MMP-9 among the experimental groups (table I);
Medium third: The expression of MMP-9 increased as the time went by (7-30 days) in 0 ppm, 5 ppm and 15 ppm F groups. There was a decrease in 50 ppm F group. At the experimental period of 7 days, there were statistically significant differences between 0 ppm and 50 ppm F groups and between 15 ppm and 50 ppm F (table I).
Activity of MMPs 2 and 9 and their proenzymes
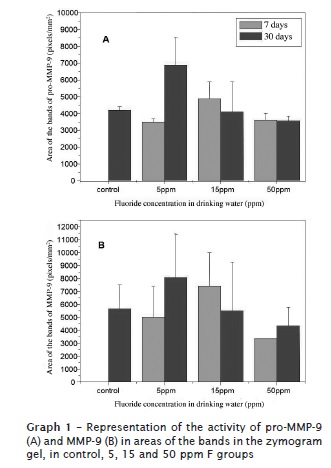
The activity of metalloproteinases 2 and 9 and their respective proenzymes were expressed in relation to the area obtained in the zymogram (analysis of the bands: number of pixels per mm2). The activity of pro-MMP-9 and MMP-9 was higher in the group treated with 5 ppm of F at 30 days than the other groups (graph 1). On the other hand, the group treated with 15 ppm of F obtained the highest activity of pro-MMP-9 and MMP-9 at 7 days. 50 ppm F group obtained the smallest values of the activity of MMP-9 (pro and active) when compared with the other groups.
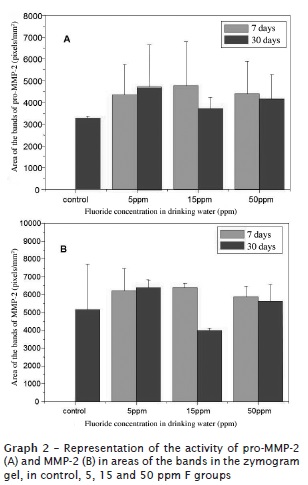
Considering to pro-MMP-2 and MMP-2, the values were very similar among the treated groups and control group at 7 and 30 days (graph 2). The exception occurred in relation to the group treated with15 ppm of F, which obtained a smaller activity than the other groups at 30 days, both for the proenzyme and active MMP-2.
Discussion
Several studies in the literature have described the event of alveolar repair after tooth extraction in rats 25,37, dogs 5 and humans 11,12. In this sense, the bone repair in alveolus after dental extraction has been adopted as a very interesting model for the study of the stages of bone repair process, from the initial events (hours and days) to late periods (months) for the identification/quantification of the cells and molecules, in addition to the expression of these genes 6,25. Some of these molecules involved in alveolar repair are MMPs 1,3. Specifically, MMPs 2 and 9 were related to the alveolar repair stages in rats and also to the cellular types present in each stage 1. The results of this present study are in agreement with those previously described 1, which also reported the interchange of the staining of MMPs 2 and 9 and the cellular types expressing them in the different stages of the alveolar repair. This expression/activity of MMPs is justified because MMPs present functions in the processes of cellular invasion/migration 28, helping the angiogenesis phenomenon 10,15,34, which brings blood support enough for the nutritional demand of the regeneration process 18. Additionally to the cellular invasion and migration, MMPs would be present in the following stage to continue the process of degradation 24 and remodeling of the matrix in the bone repair area 1,7. However, the results of this present study concerning to MMPs 2 and 9 in the groups treated with F, were different from those described by Accorsi-Mendonça et al. 1, in addition to the differences already described regarding to the control group of the aforementioned study. This may suggest a direct or indirect interference of F in MMPs 2 and 9 activity.
It is known that MMPs may be regulated in different phases, from transcription to zymogen activation 14, as well as undergoing inhibition by other molecules as TIMPs (tissue inhibitors of MMPs) 1,17 or RECK (reversion-inducing-cysteine-rich protein with Kazal motifs) protein 40,42. Therefore, the immunostaining of MMP-2 and MMP-9 itself does not guarantee that the stained enzyme have activity in the tissue because the anti-MMP antibody will be linked to an area of the enzyme present in both the pro-active and active form 1,20. Notwithstanding, the analysis by immunostaining of MMPs may show the time-space distribution of the enzymes, which is a fact of great interest. Accordingly, the quantification of the activity of MMPs by zymography is considered the most adequate method 20. Considering the aforementioned discussion, it could be understood the difference in the results of this present study between the immunostaining of MMP-9 and its activity measured by zymography. Such results are not conflicting; yet they are complementary.
The regulation and inhibition of MMPs 2 and 9 are related to the processes of blood clot resorption, as well as its replacement by connective tissue and following by bone tissue formation 10. In vitro studies have demonstrated the regulation of the activation of MMP-2 activation at the beginning of osteogenesis 1 and osteoblastic differentiation 42 through other molecules as MMPs 9 and 14, RECK and TIMP-2. Other study, conducted with knockout mice, on MMP-2 and MMP-9, respectively, showed the importance of both enzymes in the structural properties of bone tissue 24. These findings may justify the differences found in the staining for MMP-9 and consequently the difference in the repair process between the treated and control groups. Other study corroborating ours is that of Basi et al. 3, in which the authors described a delay in the alveolar repair process of rats treated with zoledronic acid associated to the increasing of the expression of MMP-9. In this same study 3 the authors suggested the increasing in the activity of MMP-9 and the increasing of the number of clasts or even in the activity of these clasts, knowing that MMP-9 is mainly expressed on osteoclast surface and in neoformation sites 29,36. The findings also indicate the staining of cells similar to clasts for MMP-9, confirming the suspect of several authors 3,29,36. The staining of MMP-9 in cells similar to macrophages, obtained in this present study, is in agreement with the study of De Jong et al. 10, who through immunohistochemical confirmed its expression in macrophages.
Conclusion
The results suggested an effect of fluoride in the activity of MMPs 2 and 9 at the initial period of the alveolar repair, which may be associated to the process of blood clot replacement and consequently to a delay in the bone repair.
Acknowledegments
We thank the collaboration of Silvana Pasetto (DDS, MsC, PhD), of Danielle Ceolin (laboratory technician), Aline L. Leite and Tatiana Furlani (undergraduates). Also, we thank the support of Fapesp (Grants #06/06430-3, #07/00494-2 and #08/09926-5) and CNPq (Grant 472798/2008-1).
References
1. Accorsi-Mendonça T, Paiva KB, Zambuzzi WF, Cestari TM, Lara VS, Sogayar MC et al. Expression of matrix metalloproteinases-2 and -9 and RECK during alveolar bone regeneration in rat. J Mol Histol. 2008 Apr;39(2):201-8. [ Links ]
2. Allori AC, Sailon AM, Warren SM. Biological basis of bone formation, remodeling, and repair – part I: biochemical signaling molecules. Tissue Eng Part B Rev. 2008 Sep;14(3):259-73.
3. Basi DL, Hughes PJ, Thumbigere-Math V, Sabino M, Mariash A, Lunos SA et al. Matrix metalloproteinase-9 expression in alveolar extraction sockets of zoledronic acid-treated rats. J Oral Maxillofac Surg. 2011 Nov;69(11):2698-707.
4. Boivin G, Chavassieux P, Chapuy MC, Baud CA, Meunier PJ. Skeletal fluorosis: histomorphometric findings. J Bone and Mineral Res. 1990 Mar;5(1):185-9.
5. Cardaropoli G, Araujo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Period. 2003 Sep;30(9):809-18.
6. Cardoso CL, Ferreira Júnior O, Carvalho PS, Dionísio TJ, Cestari TM, Garlet GP. Experimental dry socket: microscopic and molecular evaluation of two treatment modalities. Acta Cir Bras. 2011 Oct;26(5):365-72.
7. Cerri PS, Pereira-Júnior JA, Biselli NB, Sasso-Cerri E. Mast cells and MMP-9 in the lamina propria during eruption of rat molars: quantitative and immunohistochemical evaluation. J Anat. 2010 Aug;217(2):116-25.
8. Cheng K, Weng W, Wang H, Zhang S. In vitro behavior of osteoblast-like cells on fluoridated hydroxyapatite coatings. Biomaterials. 2005 Nov;26(32):6288-95.
9. Cooper LF, Zhou Y, Takebe J, Guo J, Abron A, Holmén A et al. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006 Feb;27(6):926-36.
10. De Jong PT, Tigchelaar W, Van Noorden CJ, Van der Vis HM. Polyethylene wear particles do not induce inflammation or gelatinase (MMP-2 and MMP-9) activity in fibrous tissue interfaces of loosening total hip arthroplasties. Acta Histochem. 2011 Sep;113(5):556-63.
11. Devlin H. Early bone healing events following rat molar tooth extraction. Cells Tissues Organs. 2000;167(1):33-7.
12. Devlin H, Sloan P. Early bone healing events in the human extraction socket. Int J Oral Maxillofac Surg. 2002;31(6):641-5.
13. Filanti C, Dickson GR, Di Martino D, Ulivi V, Sanguineti C, Romano P et al. The expression of metalloproteinase-2, -9, and -14 and of tissue inhibitors-1 and -2 is developmentally modulated during osteogenesis in vitro, the mature osteoblastic phenotype expressing metalloproteinase-14. J Bone Miner Res. 2000;15(11):2154-68.
14. Fridman R, Toth M, Peña D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res. 1995 Jun;55(12):2548-55.
15. Ghajar CM, George SC, Putnam AJ. Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr. 2008;18(3):251-78.
16. Gittens SA, Uludag H. Growth factor delivery for bone tissue engineering. J Drug Target. 2001;9(6):407-29.
17. Hatori K, Sasano Y, Takahashi I, Kamakura S, Kagayama M, Sasaki K. Osteoblasts and osteocytes express MMP2 and -8 and TIMP1, -2, and -3 along with extracellular matrix molecules during appositional bone formation. Anat Rec A Discov Mol Cell Evol Biol. 2004 Apr;277(2):262-71.
18. Hing KA, Best SM, Tanner KE, Bonfield W, Revell PA. Mediation of bone ingrowth in porous hydroxyapatite bone graft substitutes. J Biomed Mater Res A. 2004 Jan;68(1):187-200.
19. Kebsch M, Wilkinson M, Petocz P, Darendeliler MA. The effect of fluoride administration on rat serum osteocalcin expression during orthodontic movement. Am J Orthod Dentofacial Orthop. 2007 Apr;131(4):515-24.
20. Kupai K, Szucs G, Cseh S, Hajdu I, Csonka C, Csont T et al. Matrix metalloproteinase activity assays: importance of zymography. J Pharmacol Toxicol Methods. 2010 Mar-Apr;61(2):205-9.
21. Lowry OH, Rosenbrough NJ, Farr AL, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75.
22. Mandracchia VJ, Nelson SC, Barp EA. Current concepts of bone healing. Clin Podiatr Med Surg. 2001 Jan;18(1):55-77.
23. Mohr H. Fluoride effect on bone formation – an overview. Tandlaegebladet. 1990 Dec;94(18):761-3.
24. Nyman JS, Lynch CC, Perrien DS, Thiolloy S, OQuinn EC, Patil CA et al. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. Journal of Bone and Mineral Research. 2011;26(6):1252-60.
25. Okamoto T, Okamoto R, Alves Rezende MC, Gabrielli MF. Interference of the blood clot on granulation tissue formation after tooth extraction. Histomorphological study in rats. Braz Dent J. 1994;5(2):85-92.
26. Okamoto T, Russo MC. Wound healing following tooth extraction. Histochemical study in rats. Rev Fac Odontol Araçatuba. 1973;2(2):153-69.
27. Oliveira RC, Zambuzzi WF, Zambolin A, Silva TL, Cestari TM, Taga R et al. Marcadores bioquímicos e microscópicos como ferramentas investigativas da resposta tecidual envolvidas com a associação de osso cortical bovino/colágeno em subcutâneo de ratos. Innov Implant J. 2008 Sep;3(6):17-22.
28. Opdenakker G, Van Damme J. Chemotactic factors, passive invasion and metastasis of cancer cells. Immunol Today. 1992 Nov;13(11):463-4.
29. Page-Mccaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007 Mar;8(3):221-33.
30. Perri de Carvalho AC, Okamoto T, Garcia Jr IR. Empregos de membranas de teflon para a reparação guiada em exodontia. Rev Gaúcha Odont. 1998;46(3):127-31.
31. Qu H, Wei M. The effect of fluoride contents in fluoridated hydroxyapatite on osteoblast behavior. Acta Biomater. 2006 Jan;2(1):113-9.
32. Qu WJ, Zhong DB, Wu PF, Wang JF, Han B. Sodium fluoride modulates caprine osteoblast proliferation and differentiation. J Bone Miner Metab. 2008;26(4):328-34.
33. Reeves PG, Nielsen FH, Fahey Jr GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993 Nov;123(11):1939-51.
34. Reich A, Jaffe N, Tong A, Lavelin I, Genina O, Pines M et al. Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J Appl Physiol. 2005 Jun;98(6):2381-9.
35. Reid IR, Cundy T, Grey AB, Horne A, Clearwater J, Ames R et al. Addition of monofluorophosphate to estrogen therapy in postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2007 Jul;92(7):2446-52.
36. Reponen P, Sahlberg C, Munaut C, Thesleff I, Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994 Mar;124(6):1091-102.
37. Sato H, Takeda Y. Proliferative activity, apoptosis, and histogenesis in the early stages of rat tooth extraction wound healing. Cells Tissues Organs. 2007;186(2):104-11.
38. Souza AP, Line SRP. The biology of matrix metalloproteinases. Rev FOB. 2002;10(1):1-6.
39. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516.
40. Takahashi N, Yamana H, Yoshiki S, Roodman GD, Mundy GR, Jones SJ et al. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988 Apr;122(4):1373-82.
41. Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998 May;93(3):411-22.
42. Zambuzzi WF, Yano CL, Cavagis AD, Peppelenbosch MP, Granjeiro JM, Ferreira CV. Ascorbate-induced osteoblast differentiation recruits distinct MMP-inhibitors: RECK and TIMP-2. Mol Cell Biochem. 2009 Feb;322(1-2):143-50.
 Correspondence:
Correspondence:
Rodrigo Cardoso de Oliveira
Alameda Octávio Pinheiro Brisolla, 9-75 – Departamento de Ciências Biológicas
Faculdade de Odontologia de Bauru – Universidade de São Paulo
CEP 17012-101 – Bauru – SP – Brasil
E-mail:rodrigocardoso@usp.br
Received for publication: November 04, 2011.
Accepted for publication: December 19, 2011.













