Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.3 Joinville Jul./Set. 2012
Original Research Article
Evaluation of the level of microbial contamination and prevalence of gram-negative non-fermentative rods in dental unit waterlines
Cinthia Regiane Kotaka I ; Lourdes Botelho Garcia II; Fernanda Akemi Nakanishi Ito III ; Marcel Rodrigo Fuganti III; João Carnio IV; Jacinta Sanchez Pelayo I
II State University of Maringá – Maringá – PR – Brazil.
III School of Dentistry, State University of Londrina – Londrina – PR – Brazil.
IV Department of Periodontics, University of Florida – Gainesville – FL – USA.
ABSTRACT
Introduction: The cross infection control in dental office has received great attention from professionals and one of the critical points is the bacteriological control of water used in dental unit. Objective: To perform a microbiological evaluation of the water used in dental units, the identification of Gram-negative non-fermentative rods (GNNR) and their ability to adhere to polystyrene, and the antimicrobial activity of disinfectants on the identified strains. Material and methods: The heterotrophic bacteria count and GNNR identification were performed on water samples collected from 25 dental units (air/water syringe and reservoir). The GNNR were assessed on their capability to adhere to polystyrene and on their antimicrobial activity to the following disinfectants: sodium hypochlorite (0.06%, 0.12%, 0.25%, and0.5%) and chlorhexidine (0.03%, 0.06%, and 0.12%). Results: 88% of the air/water syringe collected samples and 68% of the reservoir collected samples were out of the potability standards. The quantity of isolated bacteria from the reservoir was lower than from the air/water syringe in 88% of the dental units. Methylobacterium spp. was found in highest percentage (19.7%) during GNNR genus isolation. There was a weak adherence to polystyrene in 85.04% of the samples. Sodium hypochlorite at 0.25%, inactivated 100% of the GNNRs in 10 minutes, while the highest tested concentration of chlorhexidine (0.12%), inactivated 98.5% of the GNNRs. Conclusion: These results provide information on the contamination problem of dental unit waterlines (DUWL) and indicate a need for treatment of the water used in dental units. The disinfection of DUWL can be performed with sodium hypochlorite at 0.25% (half the concentration recommended in the literature). However, further studies are necessary regarding DUWL frequency disinfection.
Keywords: dental equipment; water microbiological characteristics; disinfection.
Introduction
Despite the efforts to avoid cross-infection in dental office using sterilized instruments, individual protection equipment and disinfection procedures, other measures such as microbiological control of the water used in dental units are required to prevent the spread of diseases 10,11.
The quality of water in dental units is of considerable importance because both patients and dental staff are regularly exposed to water and aerosol generated by these units 20,23.
A single dental chair unit can be used in the treatment of many patients each day, and microbial contamination of specific component parts can be a significant potential source of cross-infection 10.
There are currently no official standards or legislation regarding microbial quality of dental unit waterlines (DUWL). Furthermore, until recently, there has been hardly any specific guidance from dental chair unit manufacturers on dental chair unit supply water quality 5. Actually, the responsibility for ensuring that dental chair units provide good quality water output has, by and large, been considered to rest on the shoulders of dental practitioners and/or dental clinic management.
The literature reports the use of several methods to reduce or eliminate this bacterial contamination 1,16,21. Yet, there is no standard procedure and the water used in dental units during dental treatment still shows high amounts of heterotrophic bacteria 25. This contamination can be originated from the suction of microorganisms from the patients mouth or derive from the multiplication of microorganisms contained in the water supply or in the biofilms present in DUWL 18,23,26.
The Brazilian Health Ministry Resolution n. 518 4 states that for water to be considered safe for human consumption, it should contain a maximum of 500 colony forming units per milliliter (CFU/ml), given it is free of coliforms. Both the Center for Control and Prevention of Diseases (CCD) and the American Dental Association (ADA) recommend the use of sterile water on surgical procedures with bone exposure 2, and that the water used on non-invasive procedures do not exceed 200 CFU/ml.
Dental units are equipped with a network of small bore semi-rigid plastic tubes (two to three mm) which provide water to the air/water syringe and to the handpieces 3. The water used comes from reservoirs coupled to the unit directly from tap water. Different studies show that water coming from dental units can be contaminated with microorganisms such as Pseudomonas spp., Acinetobacter spp., Burkolderia spp., Alcaligenes spp., Methylobacterium spp., Sphingomonas spp., Flavobacterium spp., and Moraxella spp., favoring biofilm formation on DUWL 1,18,28. The bacteria on the biofilm are adhered to a surface and produce extracellular polymers that ease adhesion and are even more protected from the action of antimicrobials, bacteriophages, phagocytic amoebas and from desiccation 18.
Several authors have suggested the use of disinfectants for decontamination of DUWL 1,18,21, however there is no agreement on which product is the most effective.
Considering the possible contamination of dental units due to colonization by microorganisms capable of forming biofilm, the concerns were to evaluate the bacteriological quality of water used in dental units (air/water syringe and reservoir) through the total count of heterotrophic bacteria; to isolate and identify Gram-negative nonfermentative rods (GNNR) present in water; to verify the adherence capability to polystyrene and the antimicrobial activity of different sodium hypochlorite (0.06%, 0.12%, 0.25%, 0.5%) and chlorhexidine (0.03%, 0.06%, 0.12%) concentrations against the isolated bacteria.
Material and methods
Collection of water samples
Water samples were collected from 25 dental units, being 15 from the Universities Dental Clinics (units 1 – 15); four from the Community Welfare University Center (units 16 – 19); six from the Dental Association (units 20 – 25). All samples were collected from air/water syringe and reservoir of each dental unit.
Decontamination was performed preceding collection on the external surface of the air/water syringe and reservoir through cloth friction with 70% (v/v) alcohol. A 20 to 30 seconds continuous flush was purged prior to water collection from the air/water syringe, simulating the recommended procedures for the use of equipment 6. The reservoirs were disconnected from the units for water collection, and in order to neutralize the residual chlorine from chlorine-treated water samples, approximately 100 ml of water was collected from the air/water syringe and from the reservoirs in previously sterilized flasks containing 0.1 ml 10% (w/v) sodium thiosulfate solution (Reagen, Brazil).
Total heterotrophic bacteria count
The samples were homogenized and diluted 1:10 and 1:100 in 0.9% (w/v) physiologic solution. One hundred microliters of the pure samples and dilutions were uniformly applied on the surface of plate count agar (PCA) (Difco, USA). The plates were incubated at 30ºC for 48-96 hours. The reading was carried out on the plates which showed between 30 and 300 CFU after the 48 and 96 hours incubation period. The experiments were performed in duplicate.
To evaluate whether there was a statistically significant difference regarding the contamination level of the syringe and reservoir, the Qui-square test with a 0.01 significance level was applied.
Isolation and identification of Gram-negative nonfermentative rods (GNNR)
The morphologically different colonies isolated on the PCA plates were submitted to the Gram stain. The Gram-negative bacteria were identified according to the technique described in the Manual of Clinical Microbiology 17 and through the kit NF-Prov (nonfermenters) (Newprov, Paraná, Brazil).Adhesion to inert surface (polystyrene)
Adhesion to inert surface was assessed by employing the method described by Stepanovic et al. 22 with some modification. The GNNR were incubated for 24 – 48 hours at 30ºC in Tryptic Soy Broth (TSB) (Difco, USA). The cultures were diluted 1:200 in TSB, and 200 µL of this suspension was inoculated in quadruplicate in sterile 96-well polystyrene plates (NUNC, Naperville, IL) and incubated for 24 hours at 30ºC, while negative control wells contained broth only. Then, the content of each well was aspirated and the wells were washed three times with 250 µL phosphate-buffered saline (PBS-pH 7.2). The attached bacteria were fixed with 200 µL methanol p.a (Merck, Germany) per well, and after 15 minutes the plates were emptied and left to dry. The plates were stained for five minutes with 0.2 ml 2% (w/v) Hucker crystal violet per well. Excess stain was rinsed off by placing the plate under running tap water. The plates were air-dried and the optical density (O.D.) of each well was measured at 550 nm with a Micro-ELISA Autoreader (MultiScan EX, Labsystem, Uniscience).
The cut-off O.D. (O.Dc) was defined as three standard deviations above the mean O.D. of the negative control. Strains were classified as follows: non-adherent (O.D. £ O.Dc), weakly adherent (O.Dc < O.D. £ 2 x O.Dc), moderately adherent (2 x O.Dc < O.D. £ 4 x O.Dc) and strongly adherent (4 x O.Dc < O.D.).
Evaluation of disinfectants antimicrobial activity
Sodium hypochlorite solutions of different concentrations were used: 0.06% (600 p.p.m.), 0.12% (1200 p.p.m.), 0.25% (2500 p.p.m.) and 0.5% (5000 p.p.m.). Chlorhexidine solutions were used in the following concentrations: 0.03%, 0.06%, and 0.12%.
The assay was performed in duplicate, with some modifications, and according to the technique described by Litsky and Litsky 12. The GNNR strains were inoculated in TSB and incubated for 24 – 48 hours at 30ºC.
After the incubation period, these cultures were standardized according to the turbidity, with tube one of the McFarland scale to obtain a suspension with 108 microorganisms/ml, and 1 ml of this suspension was then added to 4 ml disinfectants. The tubes were manually agitated for 1 minute and left at room temperature for 10 minutes. One hundred microliters of this mixture was uniformly applied on the surface of PCA containing neutralizer and incubated for 48 – 96 hours at 30ºC with colony counts performed after incubation. The assay performed with sodium hypochlorite had a 0.6% (w/v) sodium thiosulfate (Reagen, Brazil) neutralizer, and 0.5% (w/v) Tween 80 and 0.07% (w/v) soy lecithin (Sigma, USA) with chlorhexidine.
Results
Table I shows the heterotrophic bacteria counts found in the water collected from the reservoirs and from air/water syringes of the 25 dental units. The obtained count average in the water samples from the reservoirs was of 4.0 x 104 ± 1.0 x 105 ranging from 2.0 x 101 to 4.6 x 105 CFU/ml. The count values in the air/water syringes ranged from 1.1 x 102 to 4.6 x 105 CFU/ml, and the obtained average was 8.4 x 104 ± 1.1 x 105. According to the obtained averages of the heterotrophic bacteria count, there was a statistically significant difference regarding the contamination level of the water collected from the air/water syringe and from the reservoir when the Qui-square test with a < 0.01 was used.
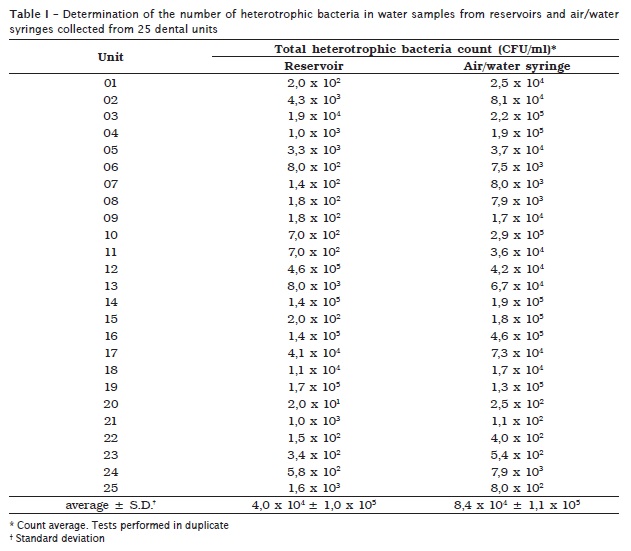
The number of isolated bacteria from the reservoir was lower than the isolated samples from the air/water syringes in most units (except for numbers 12, 19, 21, and 25).
According to the Brazilian Health Ministry Resolution n. 518 4, 88% (22/25) of the water samples from the air/water syringes and 68% (17/25) of the samples from reservoirs showed results above potability bacterial standards (table I).
GNNR isolated from air/water syringe and reservoir water samples are shown on table II. Methylobacterium spp. was the highest percentage isolated genus, (19.7%), followed by Moraxella spp. (15.2%) and Acinetobacter spp. (13.6%). Microorganisms belonging to the same genus were recovered in the reservoir and air/water syringe in 24% of dental units.

Of the studied strains 85.04% (57/67) showed weak adherence to polystyrene. Only one showed strong adherence and seven showed moderate adherence.
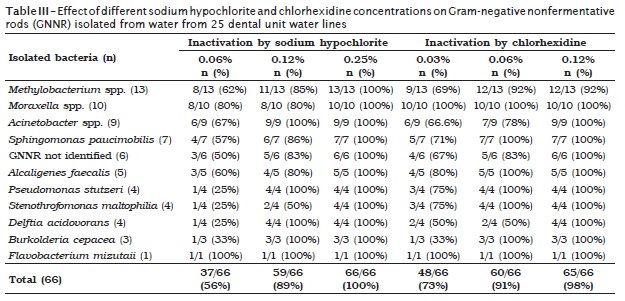
Sodium hypochlorite at 0.06% concentration inactivated 56.1% of the strains and 89.4% at 0.12%. All strains were inactivated at 0.25% (table III). 72.7% of the tested strains were inactivated with chlorhexidine at 0.03%, 90.9% at 0.06%, and 98.5% at 0.12% (table III).
Discussion
If the water used in dental unit reservoirs remains with high microbial contamination rates it will continue to be an infection source to the dentistry practice 13,28. Most DUWL microorganisms do not represent a risk to public health, but are considered opportunistic microorganisms, and therefore, liable to cause diseases to immunosuppressed patients 23.
DUWL may create favorable conditions for biofilm formation due to their small bore, wall tube imperfections, water flow, presence of minerals and organic molecules, and frequent rest periods 2,18. Under these conditions, the microorganisms present in the water used to supply the dental units multiply and form biofilm on the luminal tube surfaces 18,24.
The number of isolated bacteria in water samples from air/water syringes is usually higher than the ones isolated from reservoirs, once with the water flow through the dental unit lines there is the release of bacteria belonging to the biofilm 18,26. Hence, even when using water of good origin, if biofilm is present in DUWL the water ejected through the air/water syringe will be contaminated with bacteria belonging to the biofilm 18.
In our study, four units showed lower CFU in air/water syringe in comparison to the reservoir. This variation may be attributed to the heterogeneous distribution of bacteria in the water sample or to the age of the unit. When new, they may not show biofilm yet, and therefore, not show such different results in the water from the air/water syringe and reservoir. The same count behavior can occur if the unit is old but the lines have recently been replaced.
Variation in the total microorganism count can occur even when using appropriate culture media, temperature and incubation time since bacteria have slow growth, and some are not cultivable in the used mediums and conditions, and others are not recovered 24,25,27. These reasons, together with the difficult identification of GNNR explain why many microorganisms were isolated from the reservoir but not from the syringe and vice-versa.
The reason heterotrophic bacteria number readings were performed in 48 and 96 hours in our study is because in 48 hours we had the result of the total number of heterotrophic bacteria, but colony pigmentation only occurred after 96 hours of incubation.
We set 30ºC as the incubation temperature for our experiments since several papers reported that at this temperature there had been greater recovery of microorganisms 3,27.
This paper focused on the identification and study of GNNR. Studies performed in different countries have reported the prevalence of GNNR, opportunistic and adapted to water which proliferate and form biofilms 3,18,28. Despite the possible DUWL contamination with microorganisms from the patients mouths, oral bacteria are not usually present in dental unit waters due to the use of antiretraction valves and sterile handpieces, which control the suction of these microorganisms 26.
Bacteria found in our study were predominantly environmental organisms. Some of the bacteria identified (S. maltophilia, B. cepacea, P. stutzeri, Acinetobacter spp.) are known as opportunistic pathogens.
Martin 13 reported two cases of infections caused by P. aeruginosa acquired by immunocompromised patients after restorative dentistry treatment. In Spain, Fernández-Cuenca et al. 7 performed a survey of GNNR associated with hospital infection and found that the most important were: S. maltophilia, A. baumannii and P. aeruginosa, and Kawamura et al. 9 in Japan reported a case of recurrent bacteremia associated with venous catheter in an 11 years old girl, associated with D. acidorovans. The results of adhesion to polystyrene showed that most of the samples showed weak adherence. This is probably due to the kind of bacteria studied, since they showed a slow growth.
The results could have shown a greater number of moderately or weakly adhering bacteria if other methodologies or a longer incubation period had been used. Another factor that might have contributed to the increased number of bacteria with weak adherence was the non-addition of glucose to the culture medium (recommended in most techniques), once we strongly sought to match the conditions found in dental unit water.
The most efficient means of achieving good quality DUWL water output is through regular treatment/disinfection of DUWL with a chemical, biocide or cleaning agent that removes biofilm from DUWL effectively, and therefore, resulting in good quality water output 5,19.
Sodium hypochlorite and chlorhexidine were tested because they are products frequently used in dentistry. Chlorine compounds have been studied more extensively than any other class of chemical agents intended to control or eliminate biofilm in DUWL 1,8. Several studies indicate the use of sodium hypochlorite at 0.5% (5000 p.p.m.) to disinfect DUWLs 8,21. However, due to its high corrosive power, we tested smaller concentrations for the same purpose.
Sodium hypochlorite at 0.25% (2500 p.p.m.) was able to inactivate all tested microorganisms. We suggested the use of sodium hypochlorite at 0.25% to decontaminate DUWL, due to possible damages, both to patients and professionals and also to equipment, from the use of a more concentrated solution. However, more studies are necessary about the frequency of DUWL decontamination with sodium hypochlorite at 0.25% and its impact on equipment, on other microorganism groups (Gram-positive bacteria, Mycobacterium spp., fungi) and on the removal of already-present biofilm in the DUWLs.
The most resistant microorganisms to sodium hypochlorite at 600 p.p.m. were the P. stutzeri and bacteria that belonged to Pseudomonas genus, that is, Burkolderia spp., D. acidovorans and S. maltophilia. While S. maltophilia was the microorganism that showed the highest number of strains resistant to chlorine at 1200 p.p.m. concentration. Due to their ability to survive in aqueous mediums, these microorganisms became particularly problematic in hospital environments, once it is frequently associated with hospital infections 7,9.
Chlorhexidine is probably the most used compound in oral anti-septic compositions. New products with this active principle have been recommended by the FDA (Food and Drug Administration) to control DUWL contamination 15. Chlorhexidine shows good disinfectant activity and wide action range, nevertheless, there are disadvantages such as possible skin irritability, high costs, possibility of color changes in restorations, teeth and tongue and taste modification when continuously used 14.
Conclusion
According to our results, this paper indicates a need for treatment of the water used in dental units and provides information on the contamination problem of DUWL. The decontamination of DUWL can be performed with sodium hypochlorite at 0.25% (half the concentration recommended in the literature). However, further studies are necessary regarding DUWL frequency decontamination.
Acknowledgements
The authors thank Sergio Simões for the translation.
References
1. Abdallah SA, Khalil AI. Impact of cleaning regimes on dental water unit contamination. J Water Health. 2011;9:647-52. [ Links ]
2. Anonymous ADA. Council on Scientific Affairs. Dental unit waterlines: approaching the year 2000. J Am Dent Assoc. 1999;130:1653-63.
3. Barbeau J, Tanguay R, Faucher E, Avezard C, Trudel L, Côté L et al. Multiparametric analysis of waterline contamination in dental units. Appl Environ Microbiol. 1996;62:3954-9.
4. BRASIL. Ministério da Saúde. Secretaria de Vigilância Sanitária. Portaria n. 518, de 25 de março de 2004. Diário Oficial da União; 2004.
5. Coleman DC, ODonnell MJ, Shore AC, Swan J, Russell RJ. The role of manufacturers in reducing biofilms in dental chair waterlines. J Dent. 2007;35:701-11.
6. Fayle SA, Pollard MA. Decontamination of dental unit water systems: a review of current recommendations. Br Dent J. 1996;181:369-72.
7. Fernández-Cuenca F, López-Cortés LE, Rodríguez-Baño J. The microbiology laboratorys contribution to the surveillance and control of outbreaks caused by nonfermentative Gram-negative bacilli. Enferm Infec Microbiol Clin. 2011;29:40-6.
8. Karpay RI, Plamondon TJ, Mills SE, Dove SB. Combining periodic and continuous sodium hypoclorite treatment to control biofilms in dental unit water systems. J Am Dent Assoc. 1999;130:957-65.
9. Kawamura I, Yagi T, Hatakeyama K, Ohkura T, Ohkusu K, Takahashi Y et al. Recurrent vascular catheter-related bacteremia caused by Delftia acidovorans with different antimicrobial susceptibility profiles. J Infect Chemother. 2011;17:111-3.
10. Kumar S, Atray D, Paiwal D, Balasubramanyam G, Duraiswamy P, Kulkarni S. Dental unit waterlines: source of contamination and cross-infection. J Hosp Infect. 2010;74:99-111.
11. Lee TK, Waked EJ, Wolinsky LE, Mito RS, Daneilson RE. Controlling biofilm and microbial contamination in dental unit waterlines. J Calif Dent Assoc. 2001;29:679-84.
12. Litsky BY, Litsky W. Investigations on decontamination of hospital surfaces by the use of disinfectant-detergents. Am J Public Health. 1968;58:534-43.
13. Martin MV. The significance of the bacterial contamination of dental unit water systems. Br Dent J. 1987;163:152-4.
14. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147-79.
15. Mills SE. The dental unit waterline controversy: defusing the mythis, defining the solutions. J Am Dent Assoc. 2000;131:1427-41.
16. Murdoch-Kinch CA, Andrews NL, Atwan S, Jude R, Gleason MJ, Molinari JA. Comparison of dental water quality management procedures. J Am Dent Assoc. 1997;128:1235-43.
17. Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. Manual of Clinical Microbiology. 8. ed. Washington, DC, USA: American Society of Microbiology; 2003.
18. ODonnell MJ, Boyle MA, Russell RJ, Coleman DC. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011;6:1209-26.
19. ODonnell MJ, Shore AC, Russell RJ, Coleman DC. Optimisation of the long-term efficacy of dental chair waterline disinfection by the identification and rectification of factors associated with waterline disinfection failure. J Dent. 2007;35:438-51.
20. Pankhurst CL, Philpott-Howard JN. The microbiological quality of water in dental chair units. J Hosp Infect. 1993;23:167-74.
21. Puttaiah R, Karpay RI, Fabre C, Sherman LR, Nemeth JF, Mills SE et al. Dental unit water line treatment with sodium hypoclorite and acetic acid. Microchem J. 1998;59:333-40.
22. Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphyloccocal biofilm formation. J Microbiol Methods. 2000;40:175-9.
23. Szymanska J, Sitkowska J, Dutkiewicz J. Microbial contamination of dental unit waterlines. Ann Agric Environ Med. 2008;15:173-9.
24. Tall BD, Williams HN, George KS, Gray RT, Walch M. Bacterial succession within a biofilm in water supply lines of dental air-water syringes. Can J Microbiol. 1995;41:647-54.
25. Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martim MV, Marsh PD. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66:3363-7.
26. Whitehouse RLS, Peters E, Lizotte J, Lilge C. Influence of biofilms on microbial contamination in dental unit water. J Dent. 1991;19:290-5.
27. Williams HN, Quinby H, Romberg E. Evaluation and use of a low nutrient medium and reduced incubation temperature to study bacterial contamination in the water supply of dental units. Can J Microbiol. 1994;40:127-31.
28. Williams JF, Johnston AM, Johnson B, Huntington MK, Mackenzie CD, Path MRC. Microbial contamination of dental unit waterlines: prevalence, intensity and microbiological characteristics. J Am Dent Assoc. 1993;124:59-65.
 Correspondence:
Correspondence:
João Carnio
Av. Adhemar Pereira de Barros, 131 – Jardim Bela Suíça
CEP 86050-190 – Londrina – PR – Brasil
E-mail:jcarnio@onda.com.br
Received for publication: July 21, 2011.
Accepted for publication: February 07, 2012.













