Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.3 Joinville Jul./Set. 2012
Original Research Article
Influence of the bleaching agent and adhesive system on the bond strength of the restorative material to intracoronary dentin
João Felipe Bonatto Bruniera I; Aline Evangelista Souza-Gabriel II; Melissa Andrea Marchesan II
II School of Dentistry, University of Ribeirão Preto – Ribeirão Preto – SP – Brazil.
ABSTRACT
Introduction: The bond strength of dentin bleached with high concentrated agents can be reestablished if the appropriate restorative material is selected. Objective:The aim of this study was to evaluate the influence of bleaching agents and adhesive system on bond strength of restorative material to intracoronary dentin. Material and methods: 60 fragments of intracoronary dentin (25 mm2) were embedded in acrylic resin and divided into 3 groups (n = 10): GI – not bleached (control), GII – 35% hydrogen peroxide (35HP), GIII – 38% hydrogen peroxide (38HP). The gel was applied for 10 min onto the dentin surface. This protocol was repeated three times at a single session. After 14 days, the groups were divided into subgroups according to the adhesive system used in the restoration: A – Self-etching (Clearfil SE Bond) and B – Total etching (Single Bond 2). The fragments were restored with Z100 Filtek resin using a bipartite matrix. After 24 h, the specimens were subjected to shear bond strength test. Data were analyzed by two-way ANOVA and Tukey test (p < 0.05). Results: The group bleached with 38HP (6.02 ± 3.95) had the highest bond strength to dentin, followed by 35HP (5.36 ± 3.54), and control group (3.11 ± 2.71) (p < 0.05), although without statistically significant differences. It was also verified a higher bond strength in the group restored with the self-etching adhesive system (6.60 ± 4.18) when compared to the total etching system (3.06 ± 1.57). Conclusion: When performing the restoration of teeth bleached with hydrogen peroxide at high concentrations (35% and 38%), self-etching adhesive system should be the first choice.
Keywords: dentin; bleaching; bond strength.
Introduction
In endodont ical ly t reated teeth, crown darkening can occur because of intrinsic alteration resulting from internal bleeding, products of tissue decomposition 12, inappropriate crown opening with the maintenance of the pulp chamber roof which retains the chromophore material 4,14,18,28, and remnants of filling material inside pulp chamber in cases of incorrect cavity e 5.
Several solutions have been proposed for the bleaching of endodontically treated teeth, such as products derived from chlorine, sodium hypochlorite, hydrogen peroxide at different concentrations, urea peroxide (carbamide peroxide) and sodium perborate, placed inside pulp cavity 8,24.
However, the possibility of side effects coming from tooth bleaching cannot be discarded 23. The enamel and dentin are altered due to the application of the bleaching agents containing hydrogen peroxide, modifying its microhardness and elasticity modulus 23. These modification may be related to the reduction of the inorganic content of the enamel 7 and organic content of the dentin 6,12, as well as to alterations in the morphology of tooth substrate 25.
Despite of the possibility of side effects, either by functional or aesthetical reasons, there would be the need for restoring bleached teeth by using different adhesive systems, among which total etching systems have been used. Accordingly, to employ this adhesive system, it is necessary the previous treatment of the surface to be restored through phosphoric acid 10, to open the dentinal tubules and to increase the contact surface, therefore enabling a better retention of the restorative material 19.
The self-etching adhesive system is another system to be used, which combines the acid and primer agents in one single step, enabling a smaller chair time because of this association and simplifying the operative steps. The self-etching agents eliminate the risk of promoting the collapse of collagen fibers 13, because there is not the washing procedure followed by the drying of the substrate after the etching agent application 26. On the other hand, this adhesive system has been questioned regarding to its ability in etching tooth enamel because of the presence of acids weaker than phosphoric acids and the great amount of mineralization of tooth enamel 10,26.
Thus, it is important to assess the bond strength of the adhesive systems employed in the restoration of endodontically treated teeth submitted to tooth bleaching, considering that this procedure provoke alterations in the tooth structures.
The aim of this study was to assess the influence of both the high-concentration bleaching agent and the adhesive system on the bond strength of the restorative material to dentin.
Material and methods
Sound human maxillary central incisors, kept in 0.1% thymol solution at 9°C were washed in tap water for 24 hours to eliminated the thymol residues and macroscopically examined with the aid of a stereoscopic magnifying glass (Leica Microsystems, Wetzlar, Germany), at x20 magnification. Exclusion criteria comprised the presence of either fracture lines or fissures in tooth crown. Therefore, 60 teeth were selected.
The central incisors were properly embedded into dental utility wax (Polidental, Cotia, SP, Brazil) and cross-sectioned at the enamel-cement junction to separate the crowns from the roots. Next, the tooth crown were cut longitudinally at mesial-distal direction, with the aid of a double-faced diamond disc (KG Sorensen, Barueri, SP, Brazil) mounted in low-speed straight handpiece (Dabi Atlante, Ribeirão Preto, SP, Brazil). Each crown hemisection was again cut with the diamond disc at the incisal, mesial, distal and surface to obtain square samples of 5 mm width, totalizing 25 mm² (5 mm x 5 mm). Therefore, 30 tooth fragments were obtained.
These fragments were embedded into self-cured acrylic resin (JET-Clássico, São Paulo, SP, Brazil) with the aid of PVC rings (1.5 cm inner diameter and 1.5 cm height), previously covered by Vaseline, so that the intracoronary dentin was turned to the external environment. After the acrylic resin curing, the rings were removed and the sample surface was flatted with the aid of 280- and 400- grit silicon carbide sandpaper (Norton; Lorena, SP, Brazil), under irrigations. Next, the dentinal surface underwent 60 standardized cycles of sanding through 1200-grit sandpaper, to obtain the smear layer, to simulate the clinical situation. The specimens were washed by 10 ml of 1% sodium hypochlorite for 10 minutes, aiming to simulate the irrigation during the biomechanical preparation of the root canals.
The specimens were then divided into three groups (n = 20) according to the treatment applied onto the coronal surface: GIII – bleaching with 38% hydrogen peroxide (started by LED-laser system), GII – bleaching with 35% hydrogen peroxide (started by LED-laser system) and GI – without tooth bleaching.
The bleaching agents used were 38% hydrogen peroxide gel (Opalescence X-tra Boost, Ultradent Products, Inc., South Jordan, UT, USA), which is mixed to the red dye at the moment of its use; and 35% hydrogen peroxide (Whiteness HP, FGM). A LED-laser system (Brightness, Kondortech, São Carlos, SP, Brazil) was used for the activation of the bleaching agent.
The bleaching procedure protocol comprised the application of the bleaching gel onto the surface of the specimen (intracoronary dentin) for 45 seconds, followed by the light application (LED-laser) for the same time and removal of the bleaching gel through aspiration, according to the protocol described by Pobbe et al. 19. This procedure was repeated three times simulating a single bleaching appointment, with a interval of 10 minutes between each application.
The specimens were stored under relative humidity at 37°C for 14 days. Following, they were subdivided into two groups according to adhesive systems to be used: Clearfil SE Bond (Kuraray, Japan) – self-etching single step; and Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA). The specimens were etched with 37% phosphoric acid for 15 seconds, washed for the same time amount and dried with absorbent paper. Both adhesive systems were then applied and light-cured according to each manufacturers instruction.
The dentinal surfaces were restored with the aid of a bipartite Teflon matrix (3 mm of inner diameter and 4 mm of height) stabilized with the aid of silicone impression material (Perfil Denso, Vigodent, Bonsucesso, RJ, Brazil), to obtain composite resin cylinders with the aforementioned measurements. The composite resin (Filtek Z100, 3M ESPE, St. Paul, MN, USA was inserted in three increments with the aid of a insertion spatula (Duflex, Rio de Janeiro, RJ, Brazil), light-cured (halogen light, Dabi Atlante, Ribeirão Preto, SP, Brazil) for 40 seconds per each increment, leaving the tip of the optical fiber of the device at 10 cm above the resin surface with the aid of customized device. Next, the silicon barrier was removed with the aid of scalpel blade, the bipartite matrix opened and the specimens kept under relative humidity at 4°C for 24 h, to be submitted to the shear bond strength test. Elapsed the 24 hours, the specimens were placed in an universal testing machine (Instron 4444, Instron Corporation, Canton-Massachusetts, USA), with load of 2 kN, fixed in a stainless steel device, enabling the force incidence at 90°, avoiding the contact with the acrylic resin basis of the specimen.
The application of the shear bond strength test was performed through a rectangular stainless steel tip, at constant speed of 0.5 mm/min up to the displacement of the restoration.
The failures were analyzed through stereoscopic magnifying glass (x40 magnificat ion) (Leica Microsystems, Wetzlar, Germany) and were classified as adhesive (dentinal surface covered by a thin layer of the adhesive material); material cohesive (dentinal surface covered by composite resin); substrate cohesive (failure in dentin); mixed (the combination between adhesive and cohesive types).
The values were obtained in kN, transformed into MPa and submitted to ANOVA and Tukey test (p < 0.05) with the aid of GraphPad InStat software (GraphPad Software Inc, San Diego, CA, USA).
Results
The statistical analysis revealed no statistically significant difference between the studied factors: bleaching (p = 0.008470) and adhesive system (p = 0.000027). There was no statistically significant difference regarding to the interaction of the factors (p = 0.832770).
The group bleached by 38% hydrogen peroxide (6,02 ± 3.95) showed the highest bond strength values to dentin, followed by the group bleached by 35% hydrogen peroxide (5.36 ± 3.54) and the control group (3.11 ± 2.71), without statistically significant differences among them (p < 0.05) (table I).
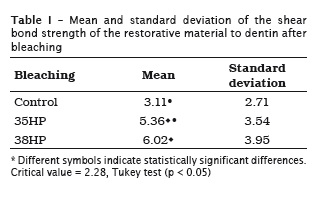
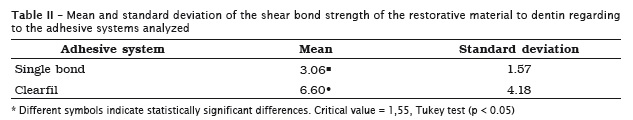
A greater bond strength was verified for the group restored with the self-etching adhesive system (6.60 ± 4.18) when compared with the total etching system (3.06 ± 1.57) (table II).
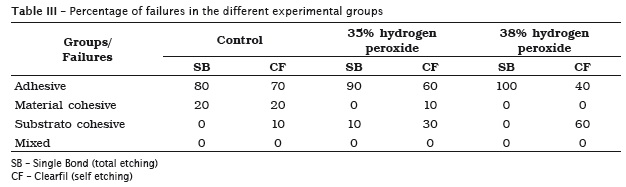
It was possible to observe the prevalence of adhesive failures in all experimental groups, except in the group bleached with 38% hydrogen peroxide and restored with Clearfil, in which substrate cohesive failures were predominantly observed, as seen in table III.
Discussion
Several studies have discussed the use of hydrogen peroxide in Dentistry because of its side effects after tooth bleaching 3,7,9,15. This present study aimed to evaluate in vitro the influence of the tooth bleaching with hydrogen peroxide gels at different concentration on the bond strength of intracoronary dentin. Hydrogen peroxide is a strong oxidant which may act on dentin by modifying its chemical and mechanical properties. The mechanism of bleaching with hydrogen peroxide has not been fully established, and some controversies still exist 11,20,27. Hydrogen peroxide is capable of generating the hydroxyl moiety (OH-) in the presence of iron salts and it has been described as accounting for tooth bleaching 3. Because of its high oxidation potential, OH- moiety acts on both the intertubular and peritubular dentin by breaking the polypeptide chains and the degrading components of the conjunctive tissue, especially collagen and hyaluronic acid, therefore acting also on the organic component of the dentin. These structural alterations increase the dentinal permeability and reduce the hardness and elasticity of the substrate 6,2.
The bond strength of the restorative material to the substrate can also be reduced due to the presence of a residual bleaching agent amount, which may interfere in the penetration of the resin into dentinal tubules. Additionally, the residual oxygen may inhibit the curing of the restorative system 22. Rotstein et al. 21 reported that the bleaching agents affect the levels of calcium, phosphor, sulfur and phosphate of the tooth tissues.
In this study, there was no stat ist ically significant difference between the groups bleached with 35% and 38% hydrogen peroxide and restored with Clearfil SE Bond and Z100. On the other hand, there was no difference between the bleached and control groups (which did not receive any bleaching procedure and were restored with the same material). A probable explanation for this similarity of behavior between the peroxide gels would be the small difference between the 35% and 38% concentrations. The period of 14 days prior to restoration may also probably contribute for these results by enabling the elimination of the residual oxygen (total or partial) from the substrate and, consequently, by not interfering in the adhesion. Barbosa et al. 1 found a similar result, because after 14 days, they did not verify differences between bleached and non-bleached specimens.
Considering to Adper Single Bond 2 (total etching system), the lower results may be attributed to its composition, comprising solvents and alcohol, which rapidly evaporates and accounts for the collapse of the collagen fibers of the dentin 17.
It is also important to consider the good behavior of the adhesive system employed (Clearfil SE Bond) on the dentin submitted to bleaching with a high concentration agent. By combining the acid and primer agents in a single flask, the system demands a small chair time and simplifies the operative steps 13. The self-etching adhesive systems eliminate the risk of promoting the collapse of the collagen fibers because the washing and drying of the substrate is not performed after the bonding agent application 26. These systems have the ability of partially dissolving the smear layer and reaching the underlying dentin, sealing the dentinal tubules 16.
The Clearfil SE Bond self-etching adhesive system contains water, organic solvents and diluents in its formula, which results in a solution fluid enough for infiltrating completely into the tooth tissue 16, unlike other adhesive systems which need a prior acid etching for its use.
In this study, it was observed the prevalence of adhesive failures for all groups, regardless of the bleaching procedure employed prior to the restorative procedure. This fact probably occurred because of the methodology employed, in which the shear bond strength acts directly on the tooth/restoration interface, separating the dentin from the restoration. It should be emphasized that both for bleached and non bleached groups there was the presence of substrate cohesive failures, demonstrating the quality of the adhesive system used. Similar results were obtained by Ferreira et al. 9.
The results of this study help to understand the mechanism of interaction of the bleaching agents on the dentinal surface. Further methods of analysis should be tested to obtain more information on the impact of the bleaching agents on the hard tissues of the tooth.
Conclusion
Based on the methodology employed and on the results obtained, it can be concluded that when performing the restoration of teeth bleached with hydrogen peroxide at high concentrations (35% and 38%), the self-etching adhesive system should be the first choice.
Acknowledgments
We thank to the São Paulo Research Foundation (FAPESP) for the f inancial support (g rant #2009/09544-8).
References
1. Barbosa CM, Sasaki RT, Florio FM, Basting RT. Influence of time on bond strength after bleaching with 35% hydrogen peroxide. J Contemp Dent Pract. 2008 Feb;9(2):81-8. [ Links ]
2. Carrasco LD, Guerisoli DMZ, Pécora JD, Fröner IC. Evaluation of dentin permeability after light activated internal dental bleaching. Dent Traumatol. 2007 Feb;23(1):30-4.
3. Carrasco-Guerisoli LD, Shiavoni RJS, Barroso JM, Guerisoli DMZ, Pécora JD, Fröner IC. Effect of different bleaching systems on the ultrastructure of bovine dentin. Dent Traumatol. 2009 Apr;25(2):176-80.
4. Carvalho EMOF, Robazza CRC, Marques JLL. Análise espectrofotométrica e visual do clareamento dental interno utilizando laser e calor como fonte catalisadora. Pesqui Odontol Bras. 2002;16(4):337-42.
5. Carrote P. Endodontic problems. Braz Dent J. 2005;198(3):127-33.
6. Chng HK, Ramli HN, Yap AUJ, Lim CT. Effect of hydrogen peroxide on intertubular dentine. J Dent. 2005 May;33(5):363-9.
7. Chng HK, Yap AUJ, Wattanapayungkul P, Sim CPC. Effect of traditional and alternative intracoronal bleaching agents on microhardness of human dentine. J Oral Rehabil. 2004 Aug;31(8):811-6.
8. Dahl JE, Pallesen U. Tooth bleaching: a critical review of the biological aspects. Crit Ver Oral Biol Med. 2003;14(4):292-304.
9. Ferreira EA, Souza-Gabriel AE, Silva-Sousa YTC, Sousa-Neto MD, Silva RG. Shear bond strength and ultrastructural interface analysis of different adhesive systems to bleached dentin. Microsc Res Tec. 2010 Jul;74(3):244-50.
10. Hegde M, Manjunath J. Bond strength of newer dentin bonding agents in different clinical situations. Oper Dent. 2011 Mar-Apr;36(2):169-76.
11. Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007 Dec;35(12):889-96.
12. Kawamoto K, Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod. 2004 Jan;30(1):45-50.
13. Kugel G, Ferrari M. The science of bonding: from first to sixth generation. J Am Dent Assoc. 2000 Jun;131(1):20-5.
14. Marchesan MA, Alfredo E, Barros F, Versiani M, Brugnera Júnior A, Sousa-Neto MD. Clareamento interno de dentes tratados endodonticamente com a utilização de LED e Laser. Odonto News. 2004;1(5):10-1.
15. Oliveira DP, Teixeira ECN, Ferraz CCR, Teixeira FB. Effect on intracoronal bleaching agents on dentin microhardness. J Endod. 2007 Apr;33(4):460-2.
16. Pashley DH, Tay FR. Aggressiveness of contemporany self-eatching adhesives. Part II: etching effects on unground enamel. Dent Mater. 2001 Sep;17:430-44.
17. Perdigão J, Lopes MM, Gomes G. In vitro bonding performance of self-etch adhesives. II. Ultramorphological evaluation. Oper Dent. 2008 Sep-Oct;(33):534-49.
18. Plotino G, Buono I, Grande NM, Pameijer CH, Somma F. Nonvital tooth bleaching a review of the literature and clinical procedures. J Endod. 2008 Apr;34(4):394-407.
19. Pobbe POS, Viapiana R, Souza-Gabriel AE, Marchesan MA, Sousa-Neto MD, Silva-Sousa YTC et al. Coronal resistance to fracture of endodontically treated teeth submitted to light-activated bleaching. J Dent. 2008 Nov;36(11):935-9.
20. Rodrigues IM, Vansan LP, Pécora JD, Marchesan MA. Permeability of different groups of maxillary teeth after 38% hydrogen peroxide internal bleaching. Braz Dent J. 2009;20(4):303-6.
21. Rotstein I, Dankner E, Goldman A, Heling I, Stabholz A, Zalkind M. Histochemical analysis of dental hard tissues following bleaching. J Endod. 1996 Jan;22(1):23-5.
22. Shinohara MS, Peris AR, Rodrigues JA, Pimenta LA, Ambrosano GM. The effect of nonvital bleaching on the shear bond strength of composite resin using three adhesive systems. J Adhes Dent. 2004:6(3):205-9.
23. Shinohara MS, Rodrigues JA, Pimenta LAF. In vitro microleakage of composite restorations after nonvital bleaching. Quintessence Int. 2001 May;32(5):413-7.
24. Souza-Gabriel AE, Vitussi LOC, Milani C, Alfredo E, Messias DCF, Silva-Sousa YTC. Effect of bleaching protocols with 38% hydrogen peroxide and post-bleaching times on dentin bond strength. Braz Dent J. 2011;22(4):317-21.
25. Sulieman M, Addy M, Macdonald E, Rees JS. The effect of hydrogen peroxide concentration on the outcome of tooth whitening: an in vitro study. J Dent. 2004 May;32(4):295-9.
26. Susin AH, Pedroso DS, Unfer DT, Rosalino TK, Marchiori JC. Resistência de união à tração de sistemas adesivos atuais. Rev Odont Ciên. 2004;19(44):144-51.
27. Tavares M, Stultz J, Newman M, Smith V, Kent R, Crpino E et al. Light augments tooth whitening with peroxide. J Am Dent Assoc. 2003 Feb;134(2):167-75.
28. Vieira C, Silva-Sousa YTC, Pessarello NM, Rached-Junior FJA, Souza-Gabriel AE. Effect of highconcentrated bleaching agents on the bond strength at dentin/resin interface and flexural strength of dentin. Braz Dent J. 2012;23(1):28-35.
 Correspondence:
Correspondence:
Aline Evangelista Souza-Gabriel
Av. Costábile Romano, n.º 2.201 – Ribeirânea
CEP 14096-900 – Ribeirão Preto – SP – Brasil
E-mail:aline.gabriel@gmail.com
Received for publication: April 07, 2012.
Accepted for publication: May 20, 2012.













