Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.9 no.4 Joinville Out./Dez. 2012
Literature Review Article
The interface between metabolic syndrome and periodontal disease
Luciene Maria Gomes Abreu I ; Fernanda Ferreira Lopes I ; Adriana de Fátima Vasconcelos Pereira I ; Antonio Luis Amaral Pereira I ; Cláudia Maria Coelho Alves I
ABSTRACT
Introduction: Metabolic syndrome (MS) is a complex pathology that combines several risk factors for cardiovascular disease. It is defined by the presence of visceral obesity, elevated triglycerides, decreased HDL, elevated blood pressure and blood glucose. The presence of at least three of these factors characterizes the syndrome. Periodontal disease (PD) is a chronic infection that produces a local and systemic inflammatory response. PD has been suggested as a possible risk factor for some of the components of MS, such as diabetes, obesity and dyslipidemia. Objective: The aim of this study was to review the literature about the possible association between periodontal disease and metabolic syndrome and to identify the components of this syndrome that may contribute to this association. Literature review: PD in the body produces a subclinical inflammatory state characterized by the release of inflammatory cytokines. Conclusion: It is plausible that these substances may contribute to the development of metabolic syndrome.
Keywords: syndrome X metabolic; periodontitis; obesity; insulin resistance; hypertension.
Introduction
Metabolic Syndrome (MS) is the denomination proposed by the World Health Organization (WHO) 42, which designates a set of risk factors for cardiovascular diseases (CVDs), such as visceral obesity, dyslipidemia, high hypertension, glucose intolerance and insulin resistance 32, which frequently are presented together.
MS definition is performed through biochemical, anthropometric and hemodynamic indicators 42.
WHO and the National Cholesterol Education Program's Adult Treatment Panel III (NCEP – ATP – III, 2001) 27 have been some of the international organizations formulating criteria for MS definition. The definition by WHO demands the evaluation of the insulin resistance. On the other hand, the definition by NCEP-ATP III does not demand the measurement of the insulin resistance, making its use ease for epidemiological studies 5. Accordingly, NCEP-ATP III criteria were employed in the execution of the I Brazilian Guideline for the Diagnosis and Treatment of Metabolic Syndrome 5, according to table I:
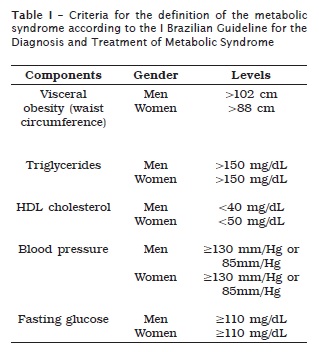
According to NCEP ATP III, an individual should be considered as having MS when he or she presents at least three of the following factors: visceral obesity, hypertriglyceridemia, HDL (reduced cholesterol), altered hypertension and fasting glycemia 27.
The International Diabetes Federation (IDF) conducted a consensus conference on MS definition, in which the ethnic differences were included in the criteria of diagnosis 1, and it emphasized the focus on the visceral obesity as the main component. The fat tissue itself is capable of producing several hormones and proteins involved in the development of diseases related to obesity 32,33.
The main difference between the guidelines from NCEP ATP III and that from IDF is that the waist circumference cutoffs for Caucasian, Blacks and Hispanics are greater in NCEP ATP III than in IDF 16.
The relatively high prevalence of the metabolic syndrome is a worldwide phenomenon that seems to be increasing in parallel with the obesity prevalence 16. In USA, 20 to 30% of the adults have MS 16. In the Japanese population, more than 20% of the population shows insulin resistance and in the adult population with diabetes mellitus type 2 the prevalence of metabolic syndrome ranges from 38 to 53%, depending on the gender and the criterion used 16.
In Brazil, most of the studies have exhibited data of population segments, such as a rural area of the semi-arid region of the state of Bahia (30%) 31; in the city of Vitória/ES (29.8%) 35; and in a Japanese-Brazilian population (57%) 14.
The insulin resistance and the proinflammatory states play a key role in the MS pathogenesis 36. Periodontitis is a chronic infectious disease affecting the world adult population, characterized by the loss of gingival insertion and bone resorption as a result of the infect ion by periodontal pathogens, such as Porphyiromonas gingivalis (Pg), Prevotella intermedia (Pi), Tannerella forsythia (Tf) and Aggregatibacter (Actinobacillus) actinomycetemcomintans (Aa) 13.
The local inflammation may initiate a systemic response by the host; therefore, subjects with periodontitis would be more prone to CVDs and other systemic diseases. Risk factors for CVDs, such as the metabolic factors may be affected by the periodontal status 29.
By considering the aforementioned discussion, the aim of this study was to analyze through a literature review the possible association between periodontal disease and metabolic syndrome and to identify the MS component that may contribute to this association.
Literature review
The periodontal disease and the components of the metabolic syndrome
Although the bacterial biofilm is necessary for the development of the periodontal disease, it alone is not enough to produce the disease. The host response, through the releasing of a large spectrum of proinflammatory mediators, is responsible for great part of the periodontal tissue destruction observed in the disease 3.
Several other factors possibly contribute to the development of the periodontal disease. Obesity 15,32,37, hypertension 30,39, dyslipidemia 10,11 and insulin resistance or diabetes 21,24-26 – components of the metabolic syndrome – has been suggested as risk factors for periodontal disease.
To study the relationship between the metabolic syndrome and its components with the periodontal disease, as well as the mechanisms behind this association, it is important for helping to clarify how other possible association would occur, that is, among the periodontal disease and the systemic conditions as diabetes type 2 and CVDs 3.
Periodontal disease and obesity
The obesity, defined by the body mass index (BMI) greater than 30.0 kg/m2, is currently a major public health problem. The obesity prevalence has substantially increased in the last decades in most of the industrialized countries; however, the underlying biological mechanisms of the association between obesity and periodontal disease have not been well known. Cytokines and hormones from the fat tissue may play an important role 32.
The fat tissue, especially the visceral type, acts as an important endocrine organ secreting several bioactive substances, such as adipocytokines. Among the most important ones are the tumor necrosis factor-alpha, leptin, adiponectin and resistin, which may modulate the periodontal response 23,33.
Leptin cont rols the appet ite, regulates the immune response and the production of inflammatory cytokines. The obesity is associated with the reduction of the sensibility to the effects of the leptin 32, which stimulates the immunological system since it increases the production of cytokines and the phagocytosis by the macrophages 33. It is a specific hormone of the adipocyte acting as a signaling molecule in the hypothalamus to complete the feedback of the lipostatic theory of weight control 23.
In periodontitis, there is a negative correlation among the levels of leptin in the gingival crevicular f luid (GCF), significantly associated with the increasing of the loss of clinical insertion 20. Two explanations have been proposed for the increase of the serum levels of leptin in periodontitis: firstly, the gingival inflammation would result in vasodilatation, which would increase the serum levels of leptin; secondly, the serum levels of leptin would increase as a defense mechanism of the body, to fight the periodontal inflammation 6.
In the inflammatory periodontal disease, the leptin regulation still needs to be further studied, especially regarding to the epidemiological association between obesity and periodontitis 32,34.
Other substance secreted by the fat tissue is the adiponectin. Unlikely to other hormones from the fat tissue, the levels of adiponectin are reduced in people with obesity, insulin resistance or diabetes type 2 32, acting as an inhibitor of the inflammatory process 18.
Experimental models have suggested that adiponectin plays a mediator role in inflammatory diseases 34, acting as a predictor of the insulin resistance and of the diabetes type 2 18.
Resistin is a specific hormone from the fat tissue recently discovered and it directly induces the insulin resistance in the muscle and liver 12.
Resistin had its role in periodontitis proved by two studies, in which the serum levels were higher in people exhibiting periodontitis than in control subjects, showing a positive correlation with bleeding on probing 12,34.
Moreover, the releasing of the tumor necrosis factor-alpha both by the liver and the periodontal tissues in response to LPS, endotoxins of gramnegative periodontal pathogens, would contribute to the insulin resistance 37. Data of a study in humans have suggested that the reduction in the serum concentration of LPS may contribute to the control of the metabolic diseases 2.
A study investigated the role of oral bacterias in the obesity epidemic. The bacterial population in the saliva of overweight women and normal weight women were measured. The percentage of Selenomonas noxia was capable of identifying 98.4% of the overweight women, suggesting the possibility that the bacterial species act as biological indicators for the development of weight gain and participate in the etiology of obesity 15.
Periodontal disease and dyslipidemia
Among the main factors involved in the increase of the lipid levels in the blood are: the genetics, a diet rich in fat, the metabolic disturbs and the lack of the physical exercises 17. One issue raised recently is whether the periodontal diseases may be a risk factor for the hyperlipidemia development 4.
Hyperlipidemia has a deregulating effect on the immune-system cells and tissue healing, increasing the susceptibility to infections, such as periodontitis 9. Current researches have been studied the association of the periodontal disease with systemic diseases, and in this relationship the alterations of lipid metabolism has been shown as a potentially inducing factor 4,9,17.
Studies showed that individuals with periodontal disease have higher serum levels of total cholesterol (TC), low density lipoprotein (LDL) cholesterol and triglycerides (TRG), when compared with periodontally healthy individuals 10,17. A study showed that women diagnosed with hyperlipidemia had significantly highest levels of the periodontal parameters than control women with normal metabolic status 4.
The alteration in the phenotype of immune cells because of the lipids and the serum elevation of proinf lammatory cytokines, such as TNF-α and IL-1β from chronic periodontitis, evidenced the bidirectional relationship between the two conditions 9.
The hyperactivity of the white blood cells caused by the hyperlipidemia increases the production of oxygen radicals, frequently associated with the periodontitis progression in adults 6,9. The reduction of the antioxidant capacity in individuals with periodontitis could facilitate the appearance of the insulin resistance 6.
It is still not clear whether the association with periodontal disease and dyslipidemia is an inter-relationship of cause-effect, that is, the periodontitis induces the highest lipid levels or the highest lipid serum levels are predisposing factors for periodontitis 9.
Periodontal disease and hypertension
Hypertension is a highly prevalent multifactorial disease affecting 30% of adults and it is one of the main causes of cardiovascular mortality and morbidity 40.
The periodontal disease may stimulate the systemic inflammation linked to CVDs. Moreover, the chronic inflammation and the inflammatory cytokines may cause endothelial dysfunction, establishing a connection between inflammation and risk for CVDs. This connection could be mediated by alterations in the vascular resistance and blood pressure (BP) 40.
The Oral Infections and Vascular Disease Epidemiology Study (INVEST) was a research designed to study the hypothesis that periodontal infections would predispose to the accelerated progression of the carotid atherosclerosis, incidence of cerebral vascular accident, myocardial infarction and other cardiovascular diseases 8.
Results of 653 patients in the United States showed that the participants in INVEST exhibited high levels of systolic and diastolic blood pressure and increase the probability of hypertension after the adjustment for conventional risk factors 8.
According to the authors, these data provide the first direct evidence of the relationship between periodontal disease and hypertension through the evaluation of the periodontal bacterial burden. Clinical parameters showing past infection, such as tooth loss, loss of insertion and probing depth were not used. There was a strong positive association between the increase of the subgingival colonization by periodontopathogens, such as A. actinomycetemcomitans, P.gingivalis, T. forsythia and T. Denticola, and the hypertension prevalence 8.
Other large-scale study in USA that employed the data from Third National Health and Nutrition Examination Survey (NHANES III) demonstrated that the gingival bleeding – a marker of periodontal inflammation – was significantly associated with high systolic blood pressure and greater chance of hypertension among adults, even after the adjustment for sociodemographic, behavioral, physiologic, and chronic disease factors 39.
Maybe both conditions may be linked to a third common factor, such as genetic predisposition. Only randomized clinical studies can reach a definitive response 30.
Periodontal disease and insulin resistance/ diabetes type 2
The world is facing a diabetes type 2 pandemic, a fact that has drawn attention not only of the scientists and health professionals, but also of the communication media 19. Previously considered as a disease from rich countries, diabetes type 2 is now a truly global affliction 19.
The International Diabetes Federation (IDF) foresees that the world incidence of diabetes among individuals aging from 20-79 years-old will increase about 70% in the next 20 years, from 194 million in 2003 to 333 million in 2025 19.
Diabetes and periodontal disease are two chronic diseases that have been considered as biologically connected 26. Löe 22 reported periodontitis as the sixth complication from diabetes. It is estimated that the prevalence of diabetic individuals would be the twice or even three times greater than that of the normal population 25.
Hyperglycemia and the format ion of the advanced glycation end-products (AGEs) are some of the several possible ways leading to the classical vascular complications of diabetes, also involved in the physiopathology of periodontitis in diabetic individuals 25.
Diabetic patients are more susceptible to develop periodontal disease because of the polymorphonuclear leukocytes and alterations in the collagen metabolism. The formation of AGEs affects the collagen stability and the vascular integrity. AGEs aggregate macrophage and monocyte receptors and they may also stimulate the releasing of interleukin- 1 and TNF-α, which provokes an increase of the susceptibility to periodontal disease 21.
Inflammatory cytokines induce the insulin resistance and the chronic inflammatory diseases, including periodontitis 24. Additionally, both TNF-α and IL-6 are produced in the fat tissue, and one third of circulating IL-6 is derived from the fat tissue, suggesting that obesity, diabetes and periodontitis be mutually related 26.
The degree of the glycemic control is an important variable in the relationship between diabetes and periodontal disease, with greater prevalence and gravity of gingival inflammation and periodontal destruction in those individuals with poor glycemic control 25.
The effect of periodontal treatment on the glycemic control of diabetic patients has been shown in interventional studies 24,38.
A current meta-analysis systematic review concluded that the periodontal therapy for diabetes type 2 patients may reduce the mean levels of glycated hemoglobin in 40% more than in control individuals that did not received any intervention 38.
Insulin resistance is present in most of the individuals with MS, strongly associated with a series of other components. However, the association with hypertension is weak. Insulin resistance is correlated with the risk of diabetes type 2 and CVDs 1.
The innate immune response is active in periodontitis, which explains the mediator role of the periodontal disease in the etiology of the insulin resistance and diabetes type 2 21.
MS components have current ly shown a considerable prevalence in populations, mainly in industrialized countries where obesity and diabetes have been already an epidemic condition. The relationship of these components with a chronic inflammatory state, common to the periodontal disease, either becomes plausible the interface among each one of these alterations and the periodontal disease or characterizes the syndrome when three or more conditions are together,
In the future, with the standardization of the diagnosis criteria of both conditions, it will be possible to establish whether this biological plausibility will be proven as a real association and therefore improve the preventive and therapeutic measurements. It is highlighted that this evidence will contribute for the prevention of CVDs.
Discussion
The evaluation of data from NHANES III revealed a greater number of individuals with periodontal disease that were also smokers. The periodontal disease participants had more metabolic syndrome (26%) than those without periodontal disease (17%). There were no differences between these groups regarding to the familiar history of diabetes or coronary artery disease or osteoporosis. The tests of correlation among the variables studied, such as gender, scholarity, and age, in the association with periodontal disease did not show significant results, except for gender (p = 0.02) 13.
Another study whose participants showed advanced periodontal disease exhibited more dysmetabolic parameters (2.5 times) than those without periodontitis. The odds ratio observed between periodontitis and metabolic syndrome in the population studied (homogenous, of high socioeconomic status and educational level) was similar to that derived from NHANES III. Most of the participants diagnosed with periodontitis were males. Unlikely to the findings of the literature, there was no significant association between periodontitis and waist circumference 28.
On the other hand, a case-control study with 302 patients exhibiting severe periodontitis and 183 healthy patients (control) had as main finding that the inflammatory and metabolic parameters were associated with their periodontal status. When compared with healthy patients, the severe periodontitis patients showed leukocytosis because of the increase of the number of circulating neutrophils and lymphocytes. Additionally, these individuals also presented dysmetabolic state characterized by the decrease of the serum levels of HDL, and increase of the insulin resistance and LDL 29.
Results from the Health 2000 Survey exhibited that the number of teeth with deep periodontal pockets (≥4 mm) was associated with BMI after the control of confounders such as gender, age, scholarity, number of teeth, smoking, and frequency of physical exercise between men and women, and also between non-smokers. The number of teeth with deep periodontal pockets was associated with the percentage of body fat (BF%) and waist circumference among non-smokers. It is concluded that obesity is at least an independent risk modifier for periodontal disease. However, because of the study design (cross-sectional), the possible causal role of the periodontal infection in the etiology of the weight gain could not be evaluated 37.
A cohort study in USA found a strong positive association between the increase of the subgingival colonizat ion by A. actinomycetemcomitans, P. gingivalis, T. forsythia and T. denticola (periodontopathogens) and the prevalence of hypertension. These associations remained positive in the subgroup gender, although the results had been stronger between men than women 40.
A study with the data from NANHES III evaluated the relationship among the different markers of inflammation/periodontal disease and blood pressure. The periodontal disease markers were associated with the results of blood pressure through regression models, with adjustment for confounders. All periodontal parameters had significant association with hypertension. Gingival bleeding was significantly associated with the increase of the systolic blood pressure and a greater chance of hypertension. When the analysis was repeated among the participants who did not use antihypertensives, the results were replicated, allowing further evidence of the association between periodontal inflammation and high blood pressure 39.
None study, however, clearly demonstrated that the periodontal disease treatment reduces the blood pressure or that the blood pressure reduction improves the periodontal status. It would be interesting to test the effect of the periodontal treatment on the efficacy of antihypertensive drugs, or vice versa. In the prospective studies of populations or patients, the relevant co-variables should be measured, including food habits, tobacco use, alcohol consumption, and dental hygiene 30.
Concerning to hyperlipidemia, there were studies reporting significant association with the levels of serum lipids and the severity of periodontal disease 9.
A study with 30 individuals with hyperlipidemia and 30 control patients evaluated the body mass index (BMI) and the clinical periodontal parameters: plaque index (PI), bleeding on probing (BP), pocket deepness (PD) and level of clinical insertion (LCI), in addition to the biochemical parameters, including triglycerides, total cholesterol, low density lipoprotein (LDL-C) and high density lipoprotein (HDL). The results exhibited that female patients with hyperlipidemia showed higher values of the periodontal parameters in comparison with control individuals. Notwithstanding, studies with larger samples in mixed populations are necessary to determine the association with hyperlipidemia and periodontal disease 4.
Other study evaluated the effect of the periodontal treatment on the control of dyslipidemia of patients in treatment with statins. All parameters of the lipids decreased after the periodontal treatment, but only the decrease of the total cholesterol (p = 0.002) and of the low density lipoprotein cholesterol (p = 0.003) reached statistically significance compared with the basal levels. This result suggested that a better periodontal health may influence the metabolic control of hyperlipidemias 9.
The bidirectional associat ion between periodontal disease and diabetes has been already well established in the literature 22,26.
The systemic inf lammat ion caused by periodontitis has an effect on the development and control of diabetes 21. Inflammatory cytokines, including TNF-α and IL-6 from periodontal disease, may induce insulin resistance 26.
As part of a v icious circle, the insul in resistance seems to modulate the inflammatory process 26. The periodontal disease affects the glucose metabolism in diabetic and non-diabetic individuals. By considering the strong causal relationship between obesity and diabetes, it can be concluded that the associations among periodontal disease, diabetes, and obesity have not been clarified yet 33.
A meta-analysis systemat ic review of interventional studies concluded that the periodontal therapy for diabetes type 2 patients is favorable and it can improve the glycemic control of these patients for at least three months 38.
The complex interaction of the inflammatory response of the host to the periodontal infections, obesity and to the alterations of the lipid levels may be responsible for the state of insulin resistance reported in individuals with periodontal disease. Such fact would explain the association among periodontal diseases, metabolic syndrome and increase of the future risk of CVDs and diabetes 28.
Conclusion
The association between periodontal disease and metabolic syndrome is compatible with the hypothesis that the chronic inflammation is an important factor in the physiopathology underlying to these condit ions. The local and systemic alterations initiated by the periodontal disease may contribute to a chronic inflammatory state, increasing the probability of developing metabolic syndrome and cardiovascular disease.
References
1. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006 May;23(5):469-80.
2. Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC et al. Energy intake is associated with endotoxemia in apparently health men. Am J Clin Nutr. 2008 May;87(5):1219-23.
3. Andriankaja OM, Sreenivasa S, Dunford R, DeNardin E. Association between metabolic syndrome and periodontal disease. Austr Dent J. 2010 Sep;55(3):252-9.
4. Awartani F, Atassi F. Evaluation of periodontal status in subjects with hyperlipidemia. J Contemp Dent Pract. 2010 Mar 1;11(2):33-40.
5. Brandão AP, Nogueira AR, Brandão AA (Eds). I Diretriz Brasileira de Diagnóstico e Tratamento da Síndrome Metabólica. Arq Bras Cardio. 2005 Apr;84 Supl 1:1-28.
6. Bullon P, Morillo JM, Ramirez-Tortosa MC, Quiles JL, Newman HB, Battino M. Metabolic syndrome and periodontitis: is oxidative stress a common link? J Dent Res. 2009 Jun;88(6):503-18.
7. D'Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani AD, Deanfield J et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J Clin Endocrinol Metab. 2008 Oct;93(10):3989-94.
8. Desvarieux M, Demmer RT, Jacobs Jr. DR, Rundek T, Boden-Albala B, Sacco RL et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. 2010 Jul;28(7):1413-21.
9. Fentoglu O, Bozkurt FY. The bi-directional relationship between periodontal disease and hyperlipidemia. Eur J Dent. 2008 Apr;2(2):142-6.
10. Fentoğlu Ö, Köroğlu BK, Hiçyılmaz H, Sert T, Özdem M, Sütçu R et al. Pro-inflammatory cytokine levels in association between periodontal disease and hyperlipidaemia. J Clin Periodontol. 2011 Jan;38(1):8-16.
11. Fentoğlu Ö, Sözen T, Öz S, Kale B, Sönmez Y, Tonguç M et al. Short-term effects of periodontal therapy as an adjunct to anti-lipemic treatment. Oral Dis. 2010 Oct;16(7):648-54.
12. Furugen R, Hayashida H, Yamaguchi N, Yoshihara A, Ogawa H, Miyazaki H et al. The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly Japanese. J Periodont Res. 2008 Oct;43(5):556-62.
13. Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67(10 Suppl):1041-9.
14. Gimeno SG, Ferreira SR, Franco LJ, Hirai AT, Matsumura L, Moisés RS. Prevalence and 7-years incidence of Type II diabetes mellitus in a Japanese- Brazilian population: an alarming public health problem. Diabetologia. 2002 Dec;45(12):1635-8.
15. Goodson JM, Guoppo D, Halem S, Carpino E. Is obesity an oral bacterial disease? J Dent Res. 2009 Jun;88(6):519-23.
16 . Grundy SM. Metabolic syndrome pandemic. Arterioscler Tromb Vasc Biol. 2008 Apr;28(4):629-39. 7. Hamissi J, Shahsavarani MT, Shahsavarani H, Sayahpour S, Hamissi H. A comparison of the serum lipid level between patients with periodontitis and healthy individuals. J Periodontol Implant Dent. 2010;2(1):29-32.
18. Hopkins T, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007 Apr;74(1):11-8.
19. International Diabetes Federation. Diabetes atlas. Bruxelas: International Diabetes Federatio; 2011 [cited 2011 July 7]. Available from: URL: http://www.eatlas.idf.org/.
20. Karthikyan BV, Pradeep AR. Gingival crevicular fluid and serum leptin: their relationship to periodontal health and disease. J Clin Periodontol. 2007 Jun;34(6):467-72.
21. Lazenby MG, Crook MA. The innate immune system and diabete mellitus: the relevance of periodontitis? A hypothesis. Clin Sci. 2010 Aug;119(10):423-9.
22. Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993 Jan;16(1):329-34.
23. Martin SS, Qasim A, Reilly MJ. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. Am Coll Cardiol. 2008 Oct;52(15):1201-10.
24. Mealey BL, Ocampo Gl. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127-53.
25. Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008 Apr;15(2):135-41.
26. Nagasawa T, Noda M, Katagiri S, Takaichi M, Takahashi Y, Wara-Aswapati N et al. Relationship between periodontitis and diabetes – importance of a clinical study to prove the vicious cycle. Inter Med. 2010;49(10):881-5.
27. National Cholesterol Education Program Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adults Treatment Panel III). JAMA. 2001 May 16;285(19):2486-97.
28. Nesbitt MJ, Reynolds MA, Shiau H, Choe K, Simonsick EM, Ferrucci L. Association of periodontitis and metabolic syndrome in the Baltimore Longitudinal study of aging. Aging Clin Exp Res. 2010 Jun;22(3):238-42.
29. Nibali L, D'Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case control study. J Clin Periodontol. 2007 Nov;34(11):931-7.
30. Odili AN, Staessen JA. Periodontal disease and hypertension: a chicken and egg story? J Hypertens. 2010 Dec;28(12):2382-3.
31. Oliveira EP, Souza ML, Lima MD. Prevalência de síndrome metabólica em uma área rural do semiárido baiano. Arq Bras Endocrinol Metab. 2006 Jun;50(3):456-65.
32. Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN, Pischon T. Obesity, inflammation and periodontal disease. J Dent Res. 2007 May;86(5):400-9.
33. Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol 2000. 2007;43:254-66.
34. Sai to T, Yamaguchi N, Shimazaki Y, Hayashida H, Yonemoto K, Doi Y et al. Serum levels of resistin and adiponectin in women with periodontitis: the Hisayama study. J Dent Res. 2008 Apr;87(4):319-22.
35. Salaroli LB, Barbosa GC, Mill JG, Molina MCB. Prevalência de síndrome metabólica em estudo de base populacional, Vitória, ES – Brasil. Arq Bras Endocrinol Metab. 2007 Oct;51(7):1143-52.
36. Samue l VT, Pe t e r s en KF, Shulma n GI . L i p i d - i n d u c e d i n s u l i n r e s i s t a n c e : unravelling the mechanism. Lancet. 2010 Jun 26;375(9733):2267-77.
37. Saxlin T, Ylostalo P, Suominen-Taipale L, Mannisto S, Knuuttila M. Association between periodontal infection and obesity: results of the Health 2000 Survey. J Clin Periodontol. 2011 Mar;38(3):236-42.
38. Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients. A systematic review and metaanalysis. Diabetes Care. 2010 Feb;33(2):421-7.
39. Tsakos G, Sabbah W, Hingorani AD, Netuveli G, Donos N, Watt RG et al. Is periodontal inflammation associated with raised blood pressure? Evidence from a National US survey. J Hypertens. 2010 Dec;28(12):2386-93.
40. Tsioufis C, Kasiakogias A, Thomopoulos C, Stefanadis C. Periodontitis and blood pressure: the concept of dental hypertension. Atherosclerosis. 2011 Nov;219(1):1-9.
41. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Geneva: World Health Organization; 1999.
 Correspondence:
Correspondence:
Cláudia Maria Coelho Alves
Programa de Pós-Graduação em Odontologia, Universidade Federal do Maranhão
Avenida dos Portugueses, s/n. – Campus do Bacanga CEP 65085-580 – São Luís – MA – Brasil
E-mail: cmcoelhoa@gmail.com













