Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.1 Joinville Jan./Mar. 2013
ORIGINAL RESEARCH ARTICLE
Lack of pulp sensitivity in maxillary canines submitted to orthodontic traction: a retrospective clinical study
Luciana Louzada FerreiraI; Eduardo Macluf FilhoII; Cláudio Luis RodriguesII; Carlos Eduardo da Silveira BuenoI; Rodrigo Sanches CunhaIII
IDepartment of Endodontics, São Leopoldo Mandic Dental Research Center – Campinas – SP – Brazil.
IIDepartment of Orthodontics, Esthetic and Orofacial Rehabilitation Center – Campinas – SP – Brazil.
IIIDepartment of Endodontics, Manitoba University – Winnipeg – Canada.
ABSTRACT
Introduction : Orthodontic movement may cause a great number of tissue alterations in the dental pulp. However, these changes may not be entirely recognized owing to the difficulty in simulating clinical situations.
Objective : The aim of this study was to clinically assess the incidence of negative pulp sensitivity to cold among maxillary canines in infraocclusion submitted to orthodontic traction.
Material and methods: Two study groups were selected: an experimental group, comprising 32 canine teeth with complete root formation that had been submitted to orthodontic traction, and a control group, comprising 32 canine teeth with complete root formation that had never been submitted to any orthodontic movement.
Results: Fourteen teeth from the experimental group showed lack of pulp sensitivity, whereas only one tooth from the control group showed negative pulp sensitivity. Fischer's exact test revealed a significant difference between the groups (p < 0.05).
Conclusion: In conclusion, the teeth that had been submitted to orthodontic traction were more likely to lack sensitivity than those that had not been submitted to the same procedure.
Keywords: canine tooth; orthodontics; dental pulp necrosis.
Introduction
The dental pulp is a singular connective tissue confined by rigid walls of mineralized tissue, in an environment that has a low tolerance to inflammation, and where the tissue is supplied by the blood vessels passing through the apical foramen.
Studies have been conducted on the effects of applying orthodontic forces to teeth in order to assess the effects of these forces on the dental pulp 1,5,9,13. However, much research has focused solely on the response of the alveolar bone or the periodontal ligament, as well as on pulpal blood flow 2,16,19.
Induced tooth movement may cause histological changes in the dental pulp, compromising its function. However, these changes may not be completely recognized owing to the difficulty involved in simulating clinical situations 18.
Applying orthodontic forces may result in pulp tissue alterations owing to the compression of the blood vessels that supply the pulp in the alveolus [8]. A light force (below 1.6 g) acts by influencing the periodontal ligament, whereas a heavy force (above 4 g) acts by partially or totally occluding blood vessels, potentially leading to degeneration or necrosis of the periodontal ligament and dental pulp 19.
Histological data demonstrate that the dental pulp is affected by orthodontic movement, and the ensuing reactions range from vascular stasis to necrosis 28. However, the specific cellular response of the pulp to orthodontic movement has not yet been investigated.
Orthodontic forces may impede pulp circulation, causing vascular congestion and pulp edema, leading to pulp damage14,27 and even necrosis in teeth submitted to traction [29]. It has been established that excessive intrusion or extrusion forces cause pulp necrosis and non-regeneration of the odontoblast layer 4.
Studies conducted in 1965 found similar characteristics between the pulp of teeth submitted to orthodontic movement and that of teeth with periodontal involvement. A radiographic analysis of teeth submitted to induced orthodontic movement showed that they seemed to age more rapidly than those that were not submitted to this procedure, demonstrating that pulp atrophy resulting from the obliteration of the root canal by reparative dentin may be involved 24.
Irreversible damage to teeth may vary from apical resorption to pulp involvement, resulting in ischemia and necrosis 1. However, it is known that pulp necrosis caused by induced tooth movement also depends on the stage of dental root development, and that teeth with an open apex have a better prognosis 10.
The aim of the present study was thus to assess clinically the lack of pulp sensitivity to cold among maxillary canines in infraocclusion submitted to non-surgical orthodontic traction.
Material and methods
This study was approved by the Ethical Committee in Research of São Leopoldo Mandic Dental Research Center, under protocol n. #2010/0165.
Sample selection
In this study, the experimental group comprised twenty four patients, 7 males and 17 females, for a total of 32 maxillary canines in infraocclusion that had been submitted to orthodontic traction. The control group comprised 16 patients, 5 males and 11 females, who had never used an orthodontic appliance or suffered any type of dental trauma, for a total of 32 canines.
Patients having a history of dental trauma, as well as teeth with incomplete apex formation or previous endodontic treatment were excluded from the study. Both groups were randomly selected among patients treated by the authors of this study in the Brazilian cities of Manaus, AM, and Campinas, SP.
Sensitivity test
The hemi-arch involved was isolated with cotton rolls. Endo-Frost (Coltene-Roeko, Germany) refrigerating gas was then applied with a cotton pellet, firstly onto the canine, then onto the lateral incisor and finally onto the first premolar. The patient's sensory response to the contact between the tooth and refrigerated cotton was recorded as either positive or negative.
Radiographic analysis
The teeth showing no pulp sensitivity were submitted to periapical radiography using periapical film (Kodak – New York, USA) and film positioners, according to the parallel technique. Radiographic analysis was then conducted to check for the occurrence of any periapical lesion, periodontal ligament thickening or pulp calcification.
Results
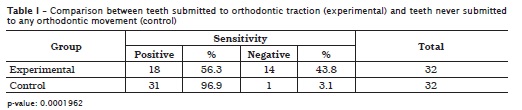
Fischer's exact test revealed a significant difference between the experimental and control groups (p < 0.05). In the control group, 3.1% of the teeth had no sensitivity, whereas those lacking sensitivity represented 43.8% in the experimental group (table I).
Discussion
Few studies have been conducted on the actual effects that induced tooth movement may have on the pulp, mainly on extrusion movements. The existing studies report the occurrence of histological alterations 5,17,26 varying from temporary degeneration – either reversible or irreversible – to pulp necrosis4,6,17, as well as circulatory or respiratory disturbances of the pulp 2,16,22.
The effects of orthodontic forces on pulpal circulation and blood flow are related to patient age, apical foramen size, dentinogenic activity and the movement itself 2,11. The results of the present study agree with those of a study reporting more significant histological disturbances in teeth with a small apical foramen 26. However, although teeth with an incompletely formed apical foramen have a reduced risk of sequelae, they are not immune to them 12. Pulp blood circulation is also altered during tooth movement 13. However, it is believed that teeth may recover from orthodontic trauma if given sufficient resting time 9,22.
Several investigators suggest that the trauma caused by induced tooth movement is permanent, and that the pulp may occasionally lose its vitality 1,10,11. Others reject this correlation7,15, or otherwise argue that necrosis of healthy teeth submitted to orthodontic movement is caused by trauma suffered by the patient and not because of the movement per se 7.
Teeth submitted to orthodontic movement may not display pulp alterations immediately. However, when teeth are submitted to forces considered as heavy, the neuropeptide calcitonin presents itself in the pulp as a response 6. Although degenerative changes may manifest long after the time of injury 25, some results show that pathologic signs appear right after the force is applied 9. Furthermore, the tissue changes are reversible if the aggression does not exceed the threshold of histological tolerance 23. No inflammatory reactions or pulp tissue alterations were observed when teeth were moved under an extrusion force of 75 g 28. Whereas some authors consider a force of 150 g 5 as an optimal force for tooth movement in adults, others suggest optimal levels ranging from 25 to 30 g 21. Hence, there is no consensus regarding the force threshold up to which tooth movement may be induced without the risk of causing pulp damage.
A study aimed at determining the effects of ischemia and necrosis following orthodontic movement in monkey teeth found that vascularity is promptly restored by collateral circulation after force removal. Nevertheless, contradictory results have been reported, varying from a temporary reversible degeneration to a permanent irreversible one, and even to pulp necrosis 4. These findings agree with those of our study, where 14 of the 32 patients from the experimental group presented negative pulp sensitivity, contrasting with the control group, which presented only one case of negative sensitivity.
It is known that different types of tooth movement have distinguishable effects on the pulp tissue. Pulp reactions were detected when orthodontic extrusion forces were compared to non-extrusion forces 17, a finding confirmed by a number of studies 1,26.
Some studies suggest that extreme tooth movements may cause changes in pulpal blood flow 5,26, but the relationship between the magnitude of vascular damage and the onset of pulp alterations remains unclear 30. On the other hand, a compromised pulpal blood flow combined with reduced apical vessel capacity during orthodontic extrusion may explain the great increase in pulp necrosis in teeth with periodontal involvement.
There is no correlation between necrosis and extrusion or orthodontic treatment time. However, in one study, more than 70% of the cases of necrosis occurred during the initial period of extrusion, highlighting the importance of exercising caution when applying orthodontic forces to traumatized teeth 3. Previous studies have also reported the occurrence of pulp necrosis during orthodontic treatment of non-traumatized teeth 20,25. Excessive tooth movement has also been reported as increasing the risk of pulp alterations, such as pulpal obliteration, in more than 20% of impacted canine teeth 30. These findings agree with those of our study, insofar as the canine teeth that had been submitted to traction in some of our patients eventually suffered pulp necrosis years after completing the orthodontic treatment.
Conclusion
Within the limitations of the applied methodology and based on the results obtained in this study, it can be concluded that maxillary canine teeth submitted to non-surgical orthodontic traction presented a greater incidence of negative sensitivity, compared to teeth not submitted to orthodontic treatment.
References
1. Anstendig HS, Kronman JH. A histologic study of pulpal reaction to orthodontic movement in dogs. Angle Orthod. 1972;42(1):50-5. [ Links ]
2. Barwick PJ, Ramsay DS. Effect of brief intrusive force on human pulpal blood flow. Am J Ortho Dentofac Orthop. 1996;110:273-9. [ Links ]
3. Bauss O, Schafer W, Sadat-Khonsari R. Influence of orthodontic extrusion on pulpal vitality of traumatized maxillary incisors. J Endod. 2010;36(2):203-7. [ Links ]
4. Butcher EO, Taylor AC. The effects of denervation and ischemia upon the teeth of the monkey. J Dent Res. 1951;30(2):265-75. [ Links ]
5. Butcher EO, Taylor AC. The vascularity of the incisor pulp of the monkey and its alteration by tooth retraction. J Dent Res. 1952;31(2):239-47. [ Links ]
6. Caviedes-Bucheli J, Moreno JO, Ardila-Pinto J, Del Toro-Carreño HR, Saltarín-Quintero H, Sierra-Tapias CL et al. The effect of orthodontic forces on calcitonin gene-related peptide expression in human dental pulp. J Endod. 2011;37(7):934-7. [ Links ]
7. Consolaro A. Orthodontic treatment does not cause pulpal necrosis. Dental Press Endod. 2011;1(1):14-20. [ Links ]
8. Dhopatkar AA, Sloan AJ, Roch WP, Cooper PR, Smith AJ. A novel in vitro culture model to investigate the reaction of the dentine-pulp complex to orthodontic force. J Orthod. 2005;32:122-32. [ Links ]
9. Grunheid T, Morbach BA, Zentner A. Pulpal cellular reactions to experimental tooth movement in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:434-41. [ Links ]
10. Guevara MJ, McClugage Junior SG. Effects of intrusive forces upon the microvasculature of the dental pulp. Angle Orthod. 1980;50(2):129-34. [ Links ]
11. Hamersky PA, Weimer AD, Taintor JF. The effect of orthodontic force application on the pulpal tissue. Am J Orthod. 1980;77(4):368-78. [ Links ]
12. Hamilton RS, Gutmann JL. Endodontic-orthodontic relationships: a review of integrated treatment planning challenges. Int Endod J. 1999;32:343-60. [ Links ]
13. Labart WA, Taintor JF, Dyer JK, Weimer AD. The effect of orthodontic forces on pulp respiration in the rat incisor. J Endod. 1980;6(9):724-7. [ Links ]
14. Marshall JA. A study of bone and tooth changes incident to experimental tooth movement and its application to orthodontic practice. Int J Orthod. 1933;19:1-17. [ Links ]
15. Massaro CS, Consolaro RB, Santamaria Junior M, Consolaro MF, Consolaro A. Analysis of the dentin-pulp complex in teeth submitted to orthodontic movement in rats. J Appl Oral Sci. 2009;17(sp issue):35-42. [ Links ]
16. McDonald F, Pitt Ford TR. Blood flow changes in permanent maxillary canines during retraction. Eur J Orthod. 1994;16:1-9. [ Links ]
17. Mostafa YA, Iskander KG, El-Mangoury NH. Iatrogenic pulpal reactions to orthodontic extrusion. Am J Orthod Dentofac Orthop. 1991;99(1):30-4. [ Links ]
18. Nixon CE, Saviano JA, King GJ, Keeling SD. Histomorphometric study of dental pulp during orthodontic tooth movement. J Endod. 1993;19(1):13-6. [ Links ]
19. Noda K, Nakamura Y, Kogure K, Nomura Y. Morphological changes in the rat periodontal ligament and its vascularity after experimental tooth movement using superelastic forces. Eur J Orthod. 2009;31:37-45. [ Links ]
20. Popp TW, Artun J, Linge L. Pulpal response to orthodontic tooth movement in adolescents: a radiographic study. Am J Orthod Dentofac Orthop. 1992;101:228-33. [ Links ]
21. Reitan TM, Vanarsdall RL. Biomechanical principles and reactions. In: Graber TM, Vanarsdall RL (eds.). Orthodontic current principles and techniques. 2 ed. St Louis: Mosby-Year Book; 1994. p. 96-105. [ Links ]
22. Sano Y, Ikawa M, Sugawara J, Horiuchi H, Mitani H. The effect of continuous intrusive force on human pulpal blood flow. Eur J Orthod. 2002;24:155-66. [ Links ]
23. Santamaria Junior M, Milagres D, Iyomasa MM, Stuani MBS, Ruellas ACO. Initial pulp changes during orthodontic movement: histomorphological evaluation. Braz Dent J. 2007;18(1):34-9. [ Links ]
24. Seltzer S, Bender IB. The dental pulp. Philadelphia: Lippincot; 1965. [ Links ]
25. Spector JK, Rothenhaus B, Herman RI. Pulpal necrosis following orthodontic therapy: report of two cases. NY State Dent J. 1974;40:30-2. [ Links ]
26. Stenvik A, Mjor A. Pulp and dentine reactions to experimental tooth intrusion: a histologic study of the initial changes. Am J Orthod. 1970;57(4):370-85. [ Links ]
27. Stuteville OH. A summary review of tissue changes incident to tooth movement. Angle Orthod. 1938;8:1-48. [ Links ]28. Sübay RK, Kaya H, Tarim B, Sübay A, Cox CF. Response of human pulpal tissue to orthodontic extrusive applications. J Endod. 2001;27(8):508-11. [ Links ]
29. Telles CS, Stuani MBS, Tavares CAE. Potencial deletério e ocorrências indesejáveis do tratamento ortodôntico. In: Todescan FF, Bottino MA. Atualização na clínica odontológica: a prática da clínica geral. São Paulo: Artes Médicas; 1996. p. 497-510. [ Links ]
30. Woloshyn H, Artun J, Kennedy DB, Joondeph DR. Pulpal and periodontal reactions to orthodontic alignment of palatally impacted canines. Angle Orthod. 1994;64(4):257-64. [ Links ]
 Corresponding author:
Corresponding author:
Luciana Louzada Ferreira
Av. Rio Negro, n. 13 – Conjunto Eldorado
CEP 69050-520 – Manaus – AM – Brasil
E-mail: lucianalouzada@ig.com.br
Received for publication: August 11, 2012
Accepted for publication: September 11, 2012













