Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.1 Joinville Jan./Mar. 2013
LITERATURE REVIEW ARTICLE
Instrumentation of dental implants: a literature review
Fabiana de Almeida CurylofoI; Lígia Araújo BarbosaI; Ana Lúcia RoselinoI; Laiza Maria Grassi FaisII; Luís Geraldo VazII
IDepartment of Diagnosis and Surgery, School of Dentistry, São Paulo State University – Araraquara – SP – Brazil.
IIDepartment of Dental Materials and Prosthesis, School of Dentistry, São Paulo State University – Araraquara – SP – Brazil.
ABSTRACT
Introduction and Objective: The aim of this study was to review the literature on the systems used to decontaminate the implant's surface. Different instruments have been proposed, but there is no agreement in the literature about which methods would be more efficient with no damage to the implant surface. It was reported the use of plastic, carbon fiber, stainless-steel and titanium curettes and also the use of other systems such as ultrasonic points with different tips, rubber cups and air abrasion.
Literature review: In most of the studies, the injury caused on the titanium surface at the time of instrumentation was examined. In others, the cell adhesion on the titanium dental implants following instrumentation of the implant surface was observed. Moreover, to enhance cleaning around implants, ultrasonic systems were recently tested.
Conclusion: Metal instruments can lead to major damage to implant surface, therefore, they are not indicated for decontamination of dental implants surfaces. Furthermore, non-metallic instruments, such as plastic curettes, rubber cups, air abrasion and some ultrasonic systems seem to be better choices to remove calculus and plaque of the sub- and supra-gingival peri-implant area. It is noteworthy that more studies evaluating the effects of these systems are required to establish best practices to be used in the treatment of patients with dental implants.
Keywords: dental implants; instrumentation; prevention and control.
Introduction
In the last decades the implant installation have become a routinely procedure for the oral rehabilitation of partially or totally edentulous patients because of its high predictability and success rates: 88% in maxilla and 93% in mandible 23. Notwithstanding, failures related to infectious process may occur in the implant therapy, therefore damaging its osseointegration 15.
The main etiologic factor of periodontal disease is dental biofilm. The bacterias within it account for the inflammatory process in the periodontal tissues 16. The sequence of bacterial colonization and biofilm formation on dental implants is similar to that occurring on the teeth 34. Moreover, it is well established that the inflammatory response to the biofilm presence in the peri-implantar tissues follow similar patterns to that of the periodontal tissues in a susceptible host 6,11,12.
Mucositis and peri-implantitis are inflammatory process developing in the tissues surrounding the osseointegrated implant and, at advanced stages, may lead to its lost 3. Considering its common points to gingivitis and periodontitis, it is understandable that the treatment of these infections follows the same guidelines recommended in the periodontal treatment. Therefore, to maintain the periodontal health around implants, a preventive system should be executed following the principles of the Support Periodontal Therapy (SPT), additionally to the adoption of intervention measurements against the pathological alterations already diagnosed 7.
The removal of the plaque and calculus on the implant surface it is necessary to achieve its long-term success 10. The mechanical procedures to clean the implant should ideally be capable of removing efficiently the bacterial deposits without altering the implant surface, which may negatively affects its biocompatibility 19.
Roughness on the titanium implant surfaces may alter the response of the surrounding soft tissues, directly influencing on the posterior dental biofilm formation and making difficult its proper removal 2,19. On the other hand, scaling procedures may also alter the oxide layer on the implant surface, which can result in the corrosion increase 25. Therefore, one should attempt to maintain the integrity of the implant surface and prosthetic components during the scaling procedures 22.
Different instruments have been proposed for the scaling of the implants. However, there is no consensus in the literature regarding which methods would be more efficient and less damaging. Based on the above discussion, the aim of this study was to report through a literature review, the main systems available for the scaling of dental titanium implants.
Literature review and discussion
Hand instruments
Instruments for cleaning dental implants should ideally be effective, cause minimum damage to titanium surface and show durability 24.
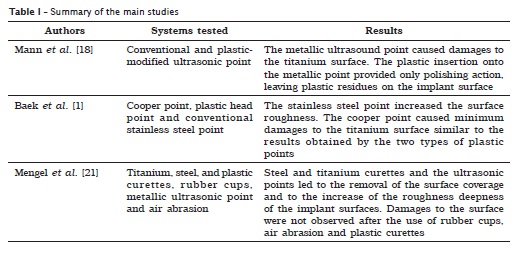
Several instruments and procedures have been proposed as alternatives to the removal of bacterial deposits of the supra- and subgingival, peri-implant area 7. The mechanical scaling performed with the aid of hand curettes of different materials is one of these alternatives 20. Among these instruments, plastic, carbon fiber, stainless-steel and titanium curettes are included. Some studies attempted to evaluate these different materials regarding to their cleaning efficacy and potential of alteration of the implant surface and prosthetic component, which could affect its biocompatibility, biofilm formation and therefore the implant longevity 5,8.
The use of plastic curettes (acetal plastic) have been largely recommended for this purpose 2,4,32. Fox et al. 8 evaluated the effects of scaling on the titanium implant surfaces, demonstrating that the plastic instruments produced the least damage than metallic instruments – stainless steel and titanium alloy –, therefore, they were recommended as instruments of choice during the routinely maintenance procedures. Other authors evaluated, in vitro, the effects of implant instrumentation on the adherence and proliferation of fibroblasts and found that the implants scaled with plastic curettes were comparable to the non-treated control implants regarding to the greatest surface compatibility to the stabilization of these cells 5.
Based on these results, the dentists prefer to use these instruments for the hygiene of implant abutments 29. However McCollum et al. 22 verified that the plastic curettes may cause vertical microgrooves on the surface of the prosthetic component, and they were not effective in removing the mature calculus. For this reason, the recommendation of the use of plastic curettes for the scaling of the implants should be carefully analyzed. Although they are the hand instruments that provoke the least alterations on the titanium surface, studies proving their efficacy and efficiency are still required.
Similarly to the plastic curettes, the carbon fiber curettes are also alternative for the scaling of implants 31. They do not significantly damage the implant surfaces and are capable of reducing the bacterial load surrounding the implants, producing an improvement of the clinical parameters of gingival index (GI), probing depth (PD) and bleeding to probe (BP) 33. Despite of these benefits, these instruments seem to leave contaminants at the site scaled, even macroscopically visible 26.
The stainless steel is other material that has been used in the curettes for implant scaling. However, researches have demonstrated that the scaling with these instruments result in risks, cuts and bruises that not only can increase the plaque retention on the titanium surface but also making its removal difficult 5,8,9.
According to Homiak et al. 9, who evaluate the effect of five different prophylaxis procedures for the instrumentation of titanium abutments, found that the stainless steel instruments provided a gouge effect, by creating a rough texture on the surfaces tested and observed by scanning electronic microscopy (SEM).
The instrumentation with these curettes was also tested regarding its effects on the cell adherence 5. It was found a significant smaller amount of adhered cells on the surfaces scaled with stainless steel curettes, although they were less irregular than those scaled with titanium alloy curettes 8, fact attributed to a possible chemical alteration on these surfaces. It is believed that stainless steel curettes may produce changes in the oxide layer of the surface or even, somehow, alter or contaminate the implant surface, which could result in a higher corrosion rate and affect its biological surface. The contact between two materials of different natures, such as stainless steel and commercially pure titanium, seems to produce even more effect than the contact of two metals of similar compositions, such as the titanium alloy and the commercially pure titanium 5.
Mengel et al. 20 examined through SEM, the vestiges left by several cleaning instruments, determining the substance amount that was removed from the implant surface. Among the instruments used for the scaling of the implants, there were titanium alloy, plastic and stainless steel curettes. The results demonstrated that the stainless steel curette left pronounced marks, indicating high substance removal; titanium curette did almost not leave marks, removing little substance; the plastic curette did not modify the implant surface, demonstrating to be adequate for the cleaning of the implant surface.
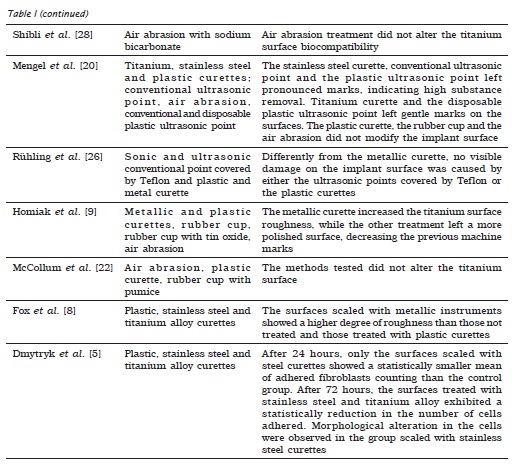
Unlikely, Mengel et al. 21 demonstrated that both the stainless steel and the titanium alloy curettes left pronounced marks on implant abutments and increased the roughness deepness on their surfaces. Souza et al. 30 also concluded that the metallic and titanium alloy curettes produced roughness on the surface, therefore being contraindicated for the scaling of the implants.
Other systems
Additionally to the use of hand instruments for implant maintenance, ultrasonic system may contribute to this purpose 13,29. Accordingly, the concerns in reducing the surrounding damages to the implants and potentiating the cleaning effect have stimulated the development of in vitro studies, which demonstrated that the implant surfaces after the use of ultrasound points, hand instruments and rubber cups, are different among each other 1,14,18,22,27. Conventional sonic and ultrasonic devices with metal tips have the advantage of being capable of removing the bacterial plaque and calculus efficiently; however, they may induce considerably modifications on the implant surface 24.
Baek et al. 1 assessed through SEM and atomic force microscopy (AFM) four different point types of a conventional ultrasound device regarding its safe and efficacy on the implant surface. The authors used copper (Cetatech, Seoul, Korea), plastic head (EMS, Nyon, Switzerland), plastic (Satelec, Merignac, France) and a conventional stainless steel point (EMS). They concluded that the stainless steel point increased the surface roughness so that it became irregular. On the other hand, the copper point caused minimum damages to the titanium surface, result similar to that obtained by the plastic points, therefore indicating the latter for the maintenance implant therapy. According to these authors, the fact that the copper point is more resistant to fracture and weariness than the other points, would be advantageous. However, further studies are still necessary.
Mann et al. 18 compared the effect of the instrumentation with a conventional ultrasound point (TFI-10) with a modified plastic point (‘SofTip', Dentsply, PA, USA) on the titanium implant surface and correlated it to the vibration movement of the instruments. The association of the profilometric and mirror laser enabled the 3D visualization of the oscillatory movement during the use of the instruments. The metallic point followed a normal pattern of oscillation. The plastic point showedlower vibration amplitude of movement, which may indicate its lack of cleaning efficacy. Different weights (100 g e 200 g) were applied onto the point during instrumentation. After their use, the titanium surface was evaluated through profilometric laser and SEM. The authors concluded that the use of ultrasonic points on the titanium surface produced higher impact, mainly when a greater weight was used, causing damage to the surface. The plastic insertion on the ultrasonic point caused minimum damages; however, it only provided a polishing action, leaving plastic residues on the implant surface.
Concerning to the use of air abrasion, a study 9 compared its effect on the implant surface with other four prophylaxis approaches – metallic and plastic curettes, rubber cup, and rubber cup associated with tin oxide. After SEM analysis, it can be concluded that when compared with the control group, the metallic curette increased the titanium surface roughness; the other treatments left a more polished surface, decreasing the machine marks on the surface.
McCollum et al. 22 evaluated the surface of the titanium prosthetic components after the exposure to air abrasion, plastic curette and rubber cups associated with pumice. When compared with the groups without treatment (control), the plastic curette created microgrooves on the surface; air abrasion left small inset bite-like aspect and the rubber cup + pumice left gentle swirl-like circles. In this study, it was also verified in vivo the plaque accumulation after the prosthetic components had been submitted to the different treatments. The prosthetic components were functional loaded for a period of seven days and the patients performed their oral hygiene normally. At the ending of that period, the components were removed and analyzed through SEM and a software evaluated the percentage of accumulated plaque. The results demonstrated that there were no statistically significant differences among the groups regarding the plaque formation surrounding the prosthetic components, fact that enabled to conclude that the methods tested did not damage the titanium surface with similar plaque formation after the treatments.
Shibli et al. 28 evaluated in vitro the growth and morphology of the fibroblasts over the surface of the titanium prosthetic components treated with air abrasion with sodium bicarbonate (Prophy-Jet). The prosthetic components were divided into two groups: without treatment (control) and with treatment (air abrasion for 30 seconds). After the treatment, the prosthetic components were incubated with fibroblastic cells for 24 hours. SEM showed that there was a reduction in the proliferation of these cells without altering their cellular morphology, indicating that the air abrasion treatment did not alter the titanium surface biocompatibility.
Therefore, the metallic instruments are more adequate in cases where there would be the need of either removing the implant coverage or making the surface smoother 17. When the treatment goal is to maintain the implant surface integrity, non-metallic instruments, rubber cups and air abrasion are the treatment of choice (table I).
Conclusion
Aiming to preserve the long-term integrity of implants, it is important to use during the prophylactic approaches, instruments which do not provoke damages to its surface. According to the literature review presented herein, the metallic instruments cause important superficial alterations and, therefore, should not be indicated for the routine scaling of implants. On the other hand, non-metallic instruments, such as plastic curettes, rubber cups, air abrasion and some ultrasonic systems seem to be better choices for removing the biofilm and calculus of the supra- and subgingival peri-implant area. It is noteworthy that further studies evaluating the clinical efficacy of these methods are necessary to define best practices to be used in the treatment of patients with dental implants.
References
1. Baek SH, Shon WJ, Bae KS, Kum KY, Lee WC, Park YS. Evaluation of the safety and efficiency of novel metallic ultrasonic scaler tip on titanium surfaces. Clin Oral Impl Res. 2011 Sep:1-6. [ Links ]
2. Balshi TJ. Hygiene maintenance procedures for patients treated with the tissue integrated prosthesis (osseointegration). Quintessence Int. 1986 Feb;17(2):95-102. [ Links ]
3. Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991 Apr-Jun;2(2):81-90. [ Links ]
4. Brough Muzzin KM, Johnson R, Carr P, Daffron P. The dental hygienist's role in the maintenance of osseointegrated dental implants. J Dent Hyg. 1988 Oct;62(9):448-53. [ Links ]
5. Dmytryk JJ, Fox SC, Moriarty JD. The effects of scaling titanium implant surfaces with metal and plastic instruments on cell attachment. J Periodontol. 1990 Aug;61(8):491-6. [ Links ]
6. Esposito M, Grusovin MG, Coulthard P, Worthington HV. Interventions for replacing missing teeth: treatment of periimplantitis. Cochrane Database Syst Rev. 2012, Issue 1. Art. No.: CD004970. DOI: 10.1002/14651858.CD004970.pub5. [ Links ]
7. Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of interventions to treat peri-implantitis: a Cochrane systematic review of randomized controlled clinical trials. Eur J Oral Implantol. 2008;1(2):111-25. [ Links ]
8. Fox SC, Moriarty JD, Kusy RP. The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol. 1990 Aug;61(8):485-90. [ Links ]
9. Homiak AW, Cook PA, DeBoer J. Effect of hygiene instrumentation on titanium abutments: a scanning electron microscopy study. J Prosthet Dent. 1992 Mar;67(3):364-9. [ Links ]
10. Hultin M, Komiyama A, Klinge B. Supportive therapy and the longevity of dental implants: a systematic review of the literature. Clin Oral Impl Res. 2007 Jun;18(3):50-62. [ Links ]
11. Karoussis IK, Brägger U, Salvi GE, Bürgin W, Lang NP. Effect of implant design on survival and success rates of titanium oral implants: a 10-year prospective cohort study of the ITI® Dental Implant System. Clin Oral Impl Res. 2004 Feb;15(1):8-17. [ Links ]
12. Karoussis IK, Müller S, Salvi GE, Heitz-mayfield LJ, Brägger U, Lang NP. Association between periodontal and peri-implant conditions: a 10-year prospective study. Clin Oral Impl Res. 2004 Feb;15(1):1-7. [ Links ]
13. Kawashima H, Sato S, Kishida M, Yagi H, Matsumoto K, Ito K. Treatment of titanium dental implants with three piezoelectric ultrasonic scalers: an in vivo study. J Periodontol. 2007 Sep;78(9):1689-94. [ Links ]
14. Koka S, Han J, Razzoog ME, Bloem TJ. The effects of two air-powder abrasive prophylaxis systems on the surface of machined titanium: a pilot study. Implant Dent. 1992;1(4):259-65. [ Links ]
15. Lindhe J, Karring T, Lang NP. Tratado de periodontia clínica e implantodontia oral. 4. ed. Rio de Janeiro: Guanabara Koogan; 2005. p. 988-97. [ Links ]
16. Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontol. 1986 May;13(5):418-30. [ Links ]
17. Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Impl Res. 2012 May;23(6):643-58. [ Links ]
18. Mann M, Parmar D, Walmsley AD, Lea SC. Effect of plastic-covered ultrasonic scalers on titanium implant surfaces. Clin Oral Impl Res. 2012 Jan;23(1):76-82. [ Links ]
19. Meffert RM. The soft tissue interface in dental implantology. J Dent Educ. 1988 Dec;52(12):810-1. [ Links ]
20. Mengel R, Buns CE, Mengel C, Flores-de-Jacoby L. An in vitro study of the treatment of implant surfaces with different instruments. Int J Oral Maxillofac Implants. 1998 Jan-Feb;13(1):91-6. [ Links ]
21. Mengel R, Meer C, Flores-de-Jacoby L. The treatment of uncoated and titanium nitride-coated abutments with different instruments. Int J Oral Maxillofac Implants. 2004 Mar-Apr;19(2):232-8. [ Links ]
22. McCollum J, O'Neal RB, Brennan WA, Van Dyke TE, Horner JA. The effect of titanium implant abutment surface irregularities on plaque accumulation in vivo. J Periodontol. 1992 Oct;63(10):802-5. [ Links ]
23. O'Roark WL. Survival rate of dental implants: an individual practitioner's anecdotal review of 25 years of experience. J Oral Implantol. 1997;23(3):90-103. [ Links ]
24. Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, van Steenberghe D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol. 1990 Mar;17(3):138-44. [ Links ]
25. Rimondini L, Farè S, brambilla E, Felloni A, consonni C, brossa F et al. The effect of surface roughness on early in vivo plaque colonization on titanium. J Periodontol. 1997 Jun;68(6):556-62. [ Links ]
26. Rühling A, Koeher T, Kreuser J, Plagman HC. Treatment of subgingival surfaces with Teflon-coated sonic and ultrasonic scaler tips and various implant curettes: an in vitro study. Clin Oral Implants Res. 1994 Mar;5(1):19-29. [ Links ]
27. Sato S, Kishida M, Ito K. The comparative effect of ultrasonic scalers on titanium surfaces: an in vitro study. J Periodontol. 2004 Sep;75(9):1269-73. [ Links ]
28. Shibli JA, Silverio KG, Martins MC, Marcantonio Jr E, Rossa Jr C. Effect of air-powder system on titanium surface on fibroblast adhesion and morphology. Implant Dent. 2003;12(1):81-6. [ Links ]
29. Silva GC, Almeida MS, Durães I. Avaliação dos instrumentos utilizados pelos cirurgiões-dentistas para higienização dos abutments de titânio. Braz Dent Sci. 2011 Jul-Dec;14(1-2):43-8. [ Links ]
30. Souza KOF, Shibli JA, Marcantonio Júnior E. Considerações clínicas sobre o tratamento das periimplantites. Rev Bras Cirur Implant. 2001 Apr-Jun;8(30):144-8. [ Links ]
31. Speelman JA, Collaert B, Klinge B. Evaluation of different methods to clean titanium abutments: a scanning microscopic study. Clin Oral Implants Res. 1992 Sep;3(3):120-7. [ Links ]
32. Stefani LA. The care and maintenance of the dental implant patient. J Dent Hyg. 1988 Oct;62(9):464-6. [ Links ]
33. Strooker H, Rohn S, Van Winkelhoff AJ. Clinical and microbiologic effects of chemical versus mechanical cleansing in professional supportive implant therapy. Int J Oral Maxillofac Implants. 1998 Nov-Dec;13(6):845-50. [ Links ]
34. Tanner A, Maiden MF, Lee K, Shulman LB, Weber HP. Dental implant infections. Clin Infect Dis. 1997 Sep;25(2):213-7. [ Links ]
 Corresponding author:
Corresponding author:
Fabiana de Almeida Curylofo
Departamento de Diagnóstico e Cirurgia – Universidade Estadual Paulista
Rua Humaitá, 1.680 – Centro
CEP 14801-903 – Araraquara – SP – Brasil
E-mail: fabianacurylofo@gmail.com
Received for publication: August 15, 2012
Accepted for publication: August 31, 2012













