Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.2 Joinville Abr./Jun. 2013
ORIGINAL RESEARCH ARTICLE
Bone healing in titanium and zirconia implants surface: a pilot study on the rabbit tibia
Gabriel MarquesI, II; Luis Eduardo Marques PadovanII; Mariza Akemi MatsumotoII; Paulo Domingos Ribeiro JúniorII; Elisa Mattias SartoriIII; Marcela ClaudinoI
IDepartment of Post-graduation, Latin American Institute of Dental Research and Education – Curitiba – PR – Brazil.
IIDepartment of Dentistry, Sagrado Coração University – Bauru – SP – Brazil.
IIIDepartment of Oral Surgery and Implantology Pathology, Camilo Castelo Branco University – Fernandópolis – SP – Brazil.
ABSTRACT
Introduction: Zirconia has been considered an alternative material to titanium for implant manufacturing, however the mechanisms regarding to bone healing in presence of zirconia implants remains poorly known.
Objective: The objective of the investigation was to evaluate the bone healing surrounding titanium and zirconia implants in rabbits after 7, 14, 30, 45 and 60 days of implant placement through histological evaluation.
Material and methods: Fifteen rabbits were used in this study and randomly subdivided into 5 groups, according to experimental periods. Titanium and zirconia implants were inserted into the right and left tibia, respectively. After healing periods of 7, 14, 30, 45 and 60 days, animals were euthanatized, the implants were removed and the samples were submitted to histological procedures.
Results: Our histological results demonstrated similar bone healing surrounding titanium and zirconia implants after 7, 14 and 30 days after implant placement. After 45 days, a trend towards to earlier bone maturation was detected, remaining after 60 days. Inflammatory infiltrate, bone resorption and foreign body reaction were not observed in any periods and groups evaluated.
Conclusion: Our findings demonstrated that zirconia and titanium presented a similar pattern of bone healing.
Keywords: dental implants; titanium; zirconia.
Introduction
Dental implants have been considered a well-accepted and predictable alternative to the rehabilitation of edentulous patients1,6. The clinical success of this treatment modality is strictly related to osseointegration, defined as a direct apposition of bone to the implant surface8.
Titanium and its alloys have been largely used in implant manufacturing due to their excellent biocompatibility and mechanical properties. Moreover, favorable results concerning to bone apposition on titanium surface are well described, demonstrating the osseointegration of titanium dental implants. Therefore, titanium has been described as a reliable implant material for long-term use in the oral cavity25.
Despite these data, several techniques and materials have been developed in order to achieve even better results, particularly as regards the aesthetic parameters. Indeed, titanium has been related to certain disadvantages such grayish color and the possible accumulation of titanium ions surrounding dental implants7 and in local lymph nodes28.
Based on these requirements, ceramic implants made of zirconia were developed, being considered a viable alternative to titanium3,16. In fact, ceramic materials such zirconia are radiopaque, extremely hard, wear resistant and its ivory color is similar to the color of natural teeth compared to the gray color of titanium, which render it an important material for use in the esthetic zone2,5,13. Generally, zirconia ceramics present a high degree of biocompatibility and exhibit minimal ion release4,13,14,23. In addition, higher fracture resilience and higher flexure strength were detected in zirconia dental implants24.
Moreover, many efforts have been made to evaluate the putative differences between zirconia and titanium surfaces concerning to osseointegration using different experimental models10-12,15,16,18.
Although several studies have been compared the osseointegration process of between titanium and zirconia implants, the putative differences concerning to biological mechanisms remain poorly known. Moreover, it would be interesting to evaluate the bone healing in presence of zirconia and titanium implants at different time points. Therefore, in the present study, we investigated the bone healing around titanium and zirconia implants in rabbits throughout a descriptive histological evaluation after 7, 14, 30, 45 and 60 days.
Material and methods
Experimental animals and implants
Fifteen New Zealand white mature male rabbits weighing 4 to 5 kg were used in this study. The animals were kept in individual cages, fed with a standard laboratory diet and given tap water ad libitum. In the sequence, rabbits were randomly subdivided into 5 groups, according to experimental periods (7, 14, 30, 45 and 60 days after implant placement). These procedures were performed under sterile conditions and the study protocol was approved by the Research Ethics Committee of the University of Sagrado Coração (116/09).
Morse taper connection implants made of commercially pure titanium (Neodent, Curitiba, Brazil) with a length of 7 mm and diameter of 3.5 mm and machined surface were placed in the right tibia of each rabbit (group 1). Implants made of zirconia ceramic with machined surface were also inserted in the left tibia of each rabbit (group 2). These implants were manufactured with a modification in the platform, characterized by an insertion of a hexagon of 1.5 mm in order to provide the implant placement. Therefore, each rabbit received 2 implants, 1 in the left tibia and 1 in the right tibia. A total of 30 implants (15 titanium implants and 15 zirconia implants) were placed.
Anesthesia, surgery and histological procedures
Prior to surgery, the shaved skin in the tibial metaphysis area was cleaned with iodine solution at the surgical and surrounding area. The animals were anaesthetized through intramuscular injection of a combination of ketamine (Ketamina Agener®; Agener União Ltda., São Paulo, SP, Brazil) (0.35 mg/kg of body weight) and xylazine (Rompum® Bayer S.A. São Paulo, SP, Brazil) (0.5 mg/kg of body weight). Incisions of approximately 3 cm in length were performed in the left and right tibiae. After dissection, the bone surface of the tibial metaphysis was exposed and one implant was placed in each tibia. Implants were placed using a progressive sequence of drills under saline cooling, according to the manufacturer’s instructions. The soft tissues were sutured in separate layers and the animals received postoperatively a single intramuscular dose of antibiotic (Pentabiótico Pequeno Porte – Fort Dodge®, Campinas, SP, Brazil) (0.1 ml/kg of body weight).
After 7, 14, 30, 45 and 60 days, animals were sacrificed by intramuscular injection of high dose of the anesthetic solution and the tibiae containing the implants were removed in terms of histological techniques. After the fixation procedures, the implants were removed and the samples were decalcified for 8 weeks in 18% EDTA, washed, dehydrated and embedded in paraffin. Serial sections with 5 μm thickness were cut and stained with goldner’s trichrome. In the sequence, all histological sections were identified with a random numerical sequence to codify experimental periods and groups during the analysis procedures performed by a single calibrated investigator with a binocular microscope (Olympus Optical Co., Tokyo, Japan).
Histological evaluation
Descriptive histological evaluation was performed in the section correspondent to central region of sockets. This evaluation includes analysis of presence of inflammatory infiltrate, formation of fibrous connective tissue, bone resorption and foreign body reaction.
Results
Our histological results demonstrated similar bone healing surrounding titanium and zirconia implants after 7, 14 and 30 days after implant placement. After 45 days, a trend towards to earlier bone maturation was detected, remaining after 60 days. Inflammatory infiltrate, bone resorption and foreign body reaction were not observed at any periods and in any groups evaluated.
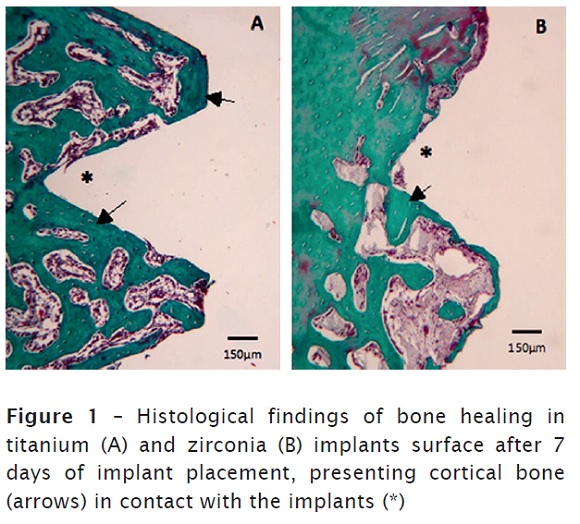
After 7 days of implant placement, cortical bone was detected in the superficial regions, referred to the cortical bone of the tibia. In the deeper portions, medullary bone was found, consisting of thin and slender newly formed bone trabeculae in the impressions of the implant threads. Furthermore, a highly vascularized fibrous tissue permeating these regions was observed, while the cortical region showed a discreet layer of connective tissue. Inflammatory cells, bone resorption and foreign body reaction were not detected in both groups. No differences between titanium and zirconia implants were found, as illustrated in figure 1A and 1B.
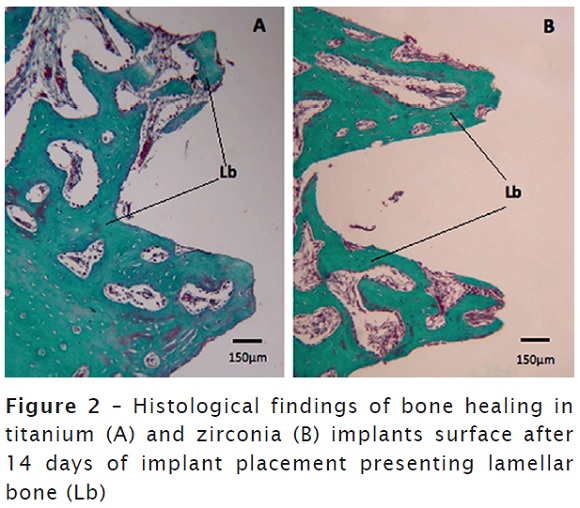
It was also detected a more dense and organized bone in the region correspondent to implant surface after 14 days. Moreover, reversion lines were detected, characterizing the maturation phase. No differences in histological findings were observed between both groups figure 2A and 2B.
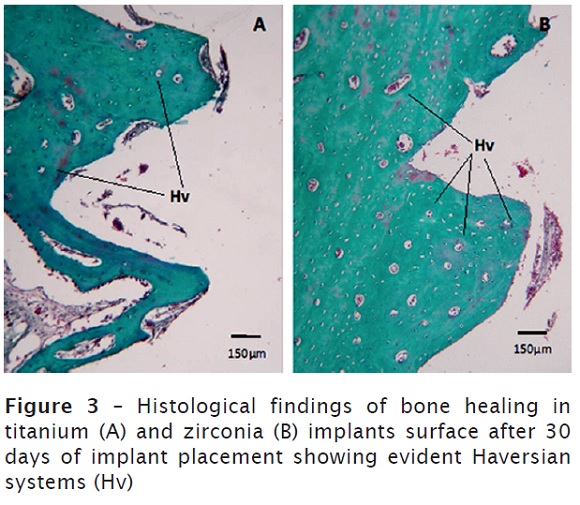
After 30 days, Haversian system was observed in the cortical bone of the region correspondent to implant surface, demonstrating the remodeling process. In addition, an intense vascularization was detected, accompanied by a strong presence of bone cells lineage. There no significant difference between titanium and zirconia implants figure 3A and 3B.
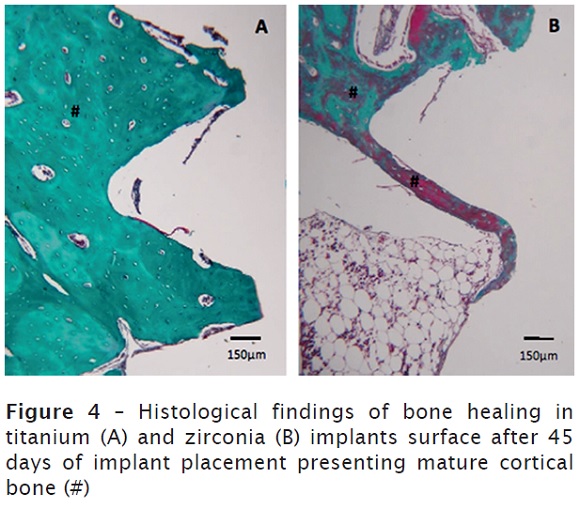
Moreover, the cortical bone detected in the superficial regions remains in the bone remodeling process at 45 days after implant placement. In the deeper portions, there was a predominantly medullar bone with thin trabeculae. No differences were observed between groups, as illustrated in figure 4A and 4B.
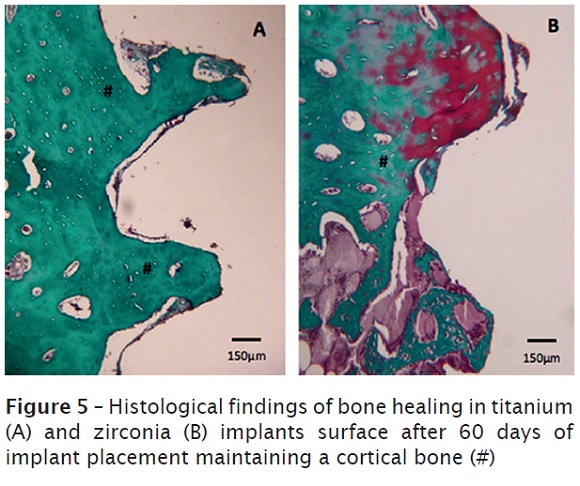
Finally, it was observed a mature bone tissue in remodeling process, characterized by reversion lines after 60 days. The corticalized aspect was more evidente in this period. As observed in other time points, no differences were detected in titanium and zirconia implants figure 5A and 5B.
Discussion
Ceramic materials have also been proposed for the use in dental implants manufacturing3,16. The applicability of these materials is associated to a wide range of advantages as mechanical characteristics and tooth-like color, allowing their indication to aesthetics regions2,5,13. Among ceramic materials available, zirconium oxide partially stabilized with yttrium (yttrium-stabilized tetragonal polycrystals [Y-TZP]) has been considered an interesting material for dental implants24. Indeed, evaluations regarding to stability of YTZP oral implants have shown that this material may be able to withstand oral forces over an extended period5,17.
Several studies have been investigated the osseointegration of zirconia implants using different experimental models10-12,15,16,18. However, it would be reasonable to evaluate the bone apposition in ceramic surfaces at different time points after implant placement. Therefore, our study demonstrated histological findings of bone healing in presence of zirconia and titanium implants placed in rabbit tibia after 7, 14, 30, 45 and 60 days.
Our histological examination revealed similar results regarding to bone healing in zirconia and titanium implants after 7, 14 e 30 days. Nevertheless, a trend of earlier bone maturation was found surrounding zirconia implants after 45 and 60 days. In fact, previous studies did not found differences in osseointegration process of zirconia implants when compared to titanium implants11,12,16,18.
Accordingly, no statistical differences were found between bone-implant contact rates in zirconia and titanium implants placed in monkeys. The mean mineralized bone-to-implant contact after 9 months of healing and 5 months of loading amounted to 72.9% for the titanium implants and 67.4% for zirconia implants16. A similar rate of bone apposition was reported after 2 and 4 weeks of zirconia and titanium implant placement using a rabbit model11. Further evaluations performed using the same experimental model in rabbits revealed comparable rates of bone apposition in the zirconia and titanium surfaces at 6 and 12 weeks of healing12. In the same way, other authors demonstrated similar bone-implant contact rates between zirconia and titanium implants placed in dogs15. Moreover, no significant differences were found regarding to histological and biomechanical results in the osseointegration of zirconia and titanium implants after 2 and 4 weeks of implant placement in rats18.
In pigs, zirconia implants showed a slight delay in osseointegration in terms of bone-implant contact rates. Zirconia implants presented rates of 59.3% and 67.1% after 4 and 12 weeks, respectively. Moreover, titanium implants revealed 64.1% and 73.6% after 4 and 12 weeks, respectively. However, no significant differences were observed20.
Our histological findings also demonstrated the absence of inflammatory infiltrate, bone resorption and foreign body reaction were not detected in any groups and periods evaluated, indicating the biocompatibility of both materials. In accordance with our data, some studies have been showed the biocompatibility of ceramic materials such as zirconia3,13,23. In monkey and rabbit models, a high degree of biocompatibility of zirconia implants was observed, characterized by high bone-implant contact rates2,23.
In fact, the biocompatibility of zirconia was previously described after implantation into muscles and bone, suggesting the absence of local and systemic effects in mice21. In vitro and in vivo experiments also revealed that bioceramics exhibited superior osteoconductive ability and biocompatibility22. Indeed, some authors related that the inflammatory response and bone resorption induced by ceramic particles were more discreet when compared to those induced by titanium alloys such Ti6A14V27.
Our data demonstrate that both implant materials can be considered highly biocompatible and osteoconductive, with a similar pattern of bone healing in both surfaces. Therefore, zirconia implants seems to have good biological properties, characterizing high biocompatible as observed in commercially pure titanium implants20,26. Accordingly, substantial evidence demonstrated similar biocompatibility and osseointegration for zirconia and titanium implants9,11,12,15-18,20,29. However, it is reasonable to consider that our sample is small, representing a limitation of our study. Other studies must be carried out to improve knowledge on this field, which may serve as a basis for development of more effective strategies for rehabilitation of edentulous patients.
Conclusion
Our findings demonstrated that zirconia and titanium presented a similar pattern of bone healing.
References
1. Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990 Winter;5(4):347-59. [ Links ]
2. Ahmad I. Yttrium-partially stabilized zirconium dioxide posts: an approach to restoring coronally compromised nonvital teeth. Int J Periodontics Restorative Dent. 1998 Oct;18(5):454-65. [ Links ]
3. Akagawa Y, Hosokawa R, Sato Y, Kamayama K. Comparison between freestanding and tooth-connected partially stabilized zirconia implants after two years’ function in monkeys: a clinical and histologic study. J Prosthet Dent. 1998 Nov;80(5):551-8.
4. Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of titanium and zirconium bone implants. Biomaterials. 1985 Mar;6(2):97-101. [ Links ]
5. Andreiotelli M, Wenz HJ, Kohal RJ. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin Oral Implants Res. 2009 Sep;20 Suppl 4:32-47. [ Links ]
6. Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997 Jun;8(3):161-72. [ Links ]
7. Bianco PD, Ducheyne P, Cuckler JM. Local accumulation of titanium released from a titanium implant in the absence of wear. J Biomed Mater Res. 1996 Jun;31(2):227-34. [ Links ]
8. Brånemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81-100. [ Links ]
9. Depprich R, Zipprich H, Ommerborn M, Naujoks C, Wiesmann HP, Kiattavorncharoen S et al. Osseointegration of zirconia implants compared with titanium: an in vivo study. Head Face Med. 2008 Dec;11(4):30. [ Links ]
10. Dubruille JH, Viguier E, Le Naour G, Dubruille MT, Auriol M, Le Charpentier Y. Evaluation of combinations of titanium, zirconia, and alumina implants with 2 bone fillers in the dog. Int J Oral Maxillofac Implants. 1999 Mar-Apr;14(2):271-7. [ Links ]
11. Hoffmann O, Angelov N, Gallez F, Jung RE, Weber FE. The zirconia implant-bone interface: a preliminary histologic evaluation in rabbits. Int J Oral Maxillofac Implants. 2008 Jul-Aug;23(4):691-5. [ Links ]
12. Hoffmann O, Angelov N, Zafiropoulos GG, Andreana S. Osseointegration of zirconia implants with different surface characteristics: an evaluation in rabbits. Int J Oral Maxillofac Implants. 2012 Mar-Apr;27(2):352-8. [ Links ]
13. Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992 Aug;68(2):322-6. [ Links ]
14. Josset Y, Oum’Hamed Z, Zarrinpour A, Lorenzato M, Adnet JJ, Laurent-Maquin D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J Biomed Mater Res. 1999 Dec 15;47(4):481-93.
15. Koch FP, Weng D, Krämer S, Biesterfeld S, Jahn-Eimermacher A, Wagner W. Osseointegration of one-piece zirconia implants compared with a titanium implant of identical design: a histomorphometric study in the dog. Clin Oral Implants Res. 2010 Mar;21(3):350-6. [ Links ]
16. Kohal RJ, Weng D, Bächle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004 Sep;75(9):1262-8. [ Links ]
17. Kohal RJ, Klaus G, Strub JR. Zirconia-implant-supported all-ceramic crowns withstand long-term load: a pilot investigation. Clin Oral Implants Res. 2006 Oct;17(5):565-71. [ Links ]
18. Kohal RJ, Wolkewitz M, Hinze M, Han JS, Bächle M, Butz F. Biomechanical and histological behavior of zirconia implants: an experiment in the rat. Clin Oral Implants Res. 2009 Apr;20(4):333-9. [ Links ]
19. Landreneau RJ, Johnson JA, Marshall JB, Hazelrigg SR, Boley TM, Curtis JJ. Clinical spectrum of paraesophageal herniation. Dig Dis Sci. 1992 Apr;37(4):537-44. [ Links ]
20. Möller B, Terheyden H, Açil Y, Purcz NM, Hertrampf K, Tabakov A et al. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: an in vivo and in vitro study. Int J Oral Maxillofac Surg. 2012 May;41(5):638-45. [ Links ]
21. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999 Jan;20(1):1-25. [ Links ]
22. Quan R, Yang D, Wu X, Wang H, Miao X, Li W. In vitro and in vivo biocompatibility of graded hydroxyapatite-zirconia composite bioceramic. J Mater Sci Mater Med. 2008 Jan;19(1):183-7. [ Links ]
23. Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003;29(1):8-12. [ Links ]
24. Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005;7 Suppl 1:S13-20. [ Links ]
25. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000 Sep-Oct;15(5):675-90. [ Links ]
26. Wang RR, Li Y. In vitro evaluation of biocompatibility of experimental titanium alloys for dental restorations. J Prosthet Dent. 1998 Oct;80(4):495-500. [ Links ]
27. Warashina H, Sakano S, Kitamura S, Yamauchi KI, Yamaguchi J, Ishiguro N et al. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials. 2003 Sep;24(21):3655-61. [ Links ]
28. Weingart D, Steinemann S, Schilli W, Strub JR, Hellerich U, Assenmacher J et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994 Dec;23(6 Pt 2):450-2. [ Links ]
29. Wenz HJ, Bartsch J, Wolfart S, Kern M. Osseointegration and clinical success of zirconia dental implants: a systematic review. Int J Prosthodont. 2008 Jan-Feb;21(1):27-36. [ Links ]
 Corresponding author:
Corresponding author:
Marcela Claudino
Instituto Latino-Americano de Pesquisa e Ensino Odontológico
Rua Jacarezinho, n. 656
CEP 80710-150 – Curitiba – PR – Brasil
E-mail: marcelaclaudino@hotmail.com
Received for publication: August 14, 2012
Accepted for publication: December 12, 2012













