Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.2 Joinville Abr./Jun. 2013
ORIGINAL RESEARCH ARTICLE
In vitro analysis of the pH alteration of the dentine after using different calcium hydroxide-based pastes
Claudio Maniglia-FerreiraI; Fabio de Almeida GomesI; Nadine Luísa Soares de Lima GuimarãesII; Marcelo de Moraes VitorianoII; Tatyana Albuquerque XimenesII; Roberto Alves dos SantosIII
I Department of Endodontics, University of Fortaleza – Fortaleza – CE – Brazil.
II School of Dentistry, University of Fortaleza – Fortaleza – CE – Brazil.
III Department of Endodontics, University of Pernambuco – Recife – PE – Brazil.
ABSTRACT
Introduction: To analyze the pH increase at the external root surface after the use of different calcium hydroxide pastes (Calen, calcium hydroxide associated with 2% chlorhexidine gel, calcium hydroxide associated with saline) with and without EDTA as chelating agent before the topical application of the intracanal medication.
Material and methods: One-hundred single-rooted extracted teeth were cleaned and shaped. They were randomly divided into six experimental groups (n = 15) and one control group (n = 10), according to the medication to be used. The teeth were kept immersed in saline solution and the pH measurements were weekly verified with the aid of a pH meter.
Results: It was verified the pH increasing at the first week in almost all groups. Only the groups in which Ca(OH)2 was associated with 2% chlorhexidine gel exhibited a significant evolution in the pH increasing over time (p = 0.0116). The use of EDTA did not result in higher pH values (p = 0.2278).
Conclusion: i) the pH increased in all associations used; ii) 2% chlorhexidine gel allowed the gradual pH increasing over time; iii) the smear layer removal did not influence on the pH increasing.
Keywords: pH alteration; intracanal medications; calcium hydroxide; chlorhexidine; Endodontics.
Introduction
The aim of the endodontic treatment is the complete removal of the pulpal tissues and/or microorganisms within this root canal system, enabling a good quality filling and consequently the regeneration/repairing of the periapical tissues9,20. For this purpose, combinations of endodontic techniques of instrumentation, irrigation/aspiration, use of intracanal medicaments and pulp cavity filling which eliminates the microorganisms within the infected root canals5,31.
The success rate of the conventional endodontic treatment reported by the literature ranges from 80% to 85%26. Therefore, the failure quite frequently occurs and demands the execution of the endodontic retreatment, because there is an increasing need of the maintenance of the teeth on the arches5. The main causes of endodontic failure are coronal infiltration, incomplete cleaning and filling, anatomic alterations and occlusal trauma9,28.
Teeth exhibiting endodontic failure have a considerable diversity of microorganisms20, 27. Compositions of calcium hydroxide-based pastes have been proposed over time and they have shown very positive antiseptic activity against these bacterial strains1,5,15,31.
Studies utilizing calcium hydroxide pastes as intracanal medications have demonstrated that at minimum intervals of seven days there is a significant reduction in the endodontic microbiota25, consume of CO211, alkalization of the dentinal tissue2,4,29 and hydrolysis of the lipopolysaccharide22. Although the antibacterial activity of calcium hydroxide is dependent on the direct contact with the bacteria23, Ørstavik and Haapasalo18 demonstrated that calcium hydroxide is not effective in eliminating the bacterias deeply colonizing the dentinal tubules. Thus, calcium hydroxide depends on other characteristics to act against the present infection, such as its capacity of increasing the pH through the dissipation of calcium ions16,29.
Because of this failure in the antibacterial activity and its low solubility, many studies have searched to find other efficient substances that could be associated with calcium hydroxide24,25,32. Chlorhexidine has been proposed as antiseptic agent in Dentistry for more than two decades and for some years as endodontic irrigating agent and intracanal medication8,24. Chlorhexidine is biocompatible and it has a large antibacterial spectrum and action against lipoteichoic acid12 associated with calcium hydroxide, demonstrating excellent results in clinical and laboratorial studies7,13-15,30.
Several studies have demonstrated the good efficacy of the association of calcium hydroxide with several vehicles19,32. Its association with chlorhexidine gel has been little studied and reported in literature regarding the releasing of hydroxyl ions. Thus, this study aimed to analyze in vitro the capacity of pH increasing of the external root surface of different associations of calcium hydroxide used as intracanal medications in extracted teeth.
Material and methods
This was an experimental, quantitative, transversal study of prospective character which aimed to analyze in vitro the pH increasing in the external surface of the root after the use of different calcium hydroxide pastes (Calen® paste, calcium hydroxide associated with 2% chlorhexidine gel and calcium hydroxide associated with saline solution), with and without the use of EDTA prior to the topical application of the intracanal medication.
Obtainment and selection of the specimens
This study was executed on 100 single-rooted natural teeht that were extracted and kept in 10% formalin solution until their use. The origin data of the specimens are seen in a donation consent form attached to the research project referred to the Ethical Committee in Research of the University of Fortaleza. The project was approved under protocol number 364/2006.
Preparation of the specimens
The cleaning and shaping procedures of the specimens were based on the crown-down technique described Maniglia-Ferreira et al13. The irrigation procedures were standardized with the use of 5 ml of 1% sodium hypochlorite (Biodinâmica, São Paulo, Brazil) at every change of instrument. The irrigation in all groups of teeht was executed with the aid of a disposable syringe (5 ml) associated with a BD needle (20X0.55 mm). All canals were dried with absorbent paper points previously to the topical application of the calcium hydroxide pastes.
Preparation and composition of the calcium hydroxide – Ca(OH)2 pastes
Calen® paste (S.S.White, Rio de Janeiro, Brazil)
Product kept in tubes with the following composition: Ca(OH)2, polyethylene glycol 800 and colophony.
Association of calcium hydroxide with 2%chlorhexidine gel (CX)
The paste was obtained by mixing Ca(OH)2 PA (Biodinâmica, São Paulo, Brazil) with 2% chlorhexidine gel (Endogel, Endosupport, Itapetininga, SP, Brazil), at the proportion of 1 ml vehicle/1 gram of powder, up to a paste consistency.
Association of calcium hydroxide with saline solution
The paste was obtained by mixing Ca(OH)2 PA (Biodinâmica, São Paulo, Brazil) with saline solution (0.9% sodium chloride) (Gaspar Viana S.A., Fortaleza, CE, Brazil), at the proportion of 1 ml vehicle/1 gram of powder, up to a paste consistency.
Study groups
After the instrumentation procedures, all teeht were randomly divided into six experimental groups of 15 specimens. The teeth of each group were filled with a different calcium hydroxide-based (Ca(OH)2) paste, according to table I. Ten specimens were used to compose the control group, in which there is no application of the intracanal medication.
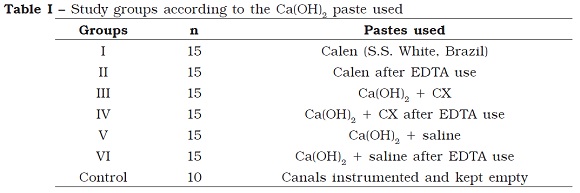
For groups II, IV and VI, EDTA was used as auxiliary chemical agent, for 4 minutes, to remove the smear layer, prior to the final irrigation.
The filling of the root canals with the different calcium hydroxide pastes was carried out with the aid of irrigation syringes for the specimens of the groups III to VI, while the specimens of groups I and II (Calen pate) a special syringe suitable for the application of this medicament was employed according to the manufacturer’s instructions.
Verification of the pH alteration
All teeth received provisional restorations with Super Bonder glue (3M do Brazil, Campinas, SP, Brazil), suspended onto individual holders in Becker flasks containing saline solution for the analysis of the pH alteration. The pH meter (Micronal® – model B474) was connected to the liquid involving the specimens of each group through its terminal portion, so-called electrode. The readings were performed at every week for 4 weeks. All values were recorded in sheets for statistical analysis.
Results
The results were statistically analyzed with the aid of Bioestat 5.0 software and did not follow the normal curve distribution. Data was submitted to Kruskal-Wallis test, with level of significance of 5%. The pH value means for the different groups and time periods studied are seen in table II.
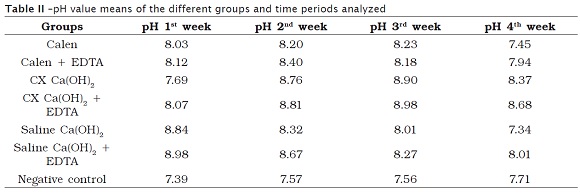
Except for group III, all materials used promoted significant increasing in pH values at the first week (p = 0.0095) in comparison with the control group which did not exhibited pH alteration at the different time periods analyzed (p = 0.3789).
Groups III and IV showed significant evolution in the pH increasing over time (p = 0.0116), differing from the other groups which demonstrated a decrease in the pH values over time. The use of EDTA did not provided higher pH values (p = 0.2278).
Discussion
The hypothesis that the diffusion of hydroxyl ions from calcium hydroxide through the dentinal tubules would increase the pH of the dental tissues was evaluated by Tronstad et al.29, by using an experimental animal model. The authors verified that the insertion of calcium hydroxide within root canal has a direct influence on the pH increase of the external root surface, mainly in areas of root resorption or incomplete root formation, making impossible the osteoclastic activity and stimulating the repairing of the local tissues. In this present study, a previous analysis of the provisional restorative materials was executed so that they did not influence on the results. Because Super Bonder glue did not show any influence on pH, it was the option of choice for the coronal and apical sealing. In a pilot study with different provisional restorative materials, we found many influences on pH, both separately and when they were applied as restoration of teeth with intracanal medication of calcium hydroxide. It is known, however, that the provisional restoration with ideal properties of neutral pH is almost impossible in Dentistry, but a substance closer to that desired conditions must be searched and if possible, found. The time intervals selected for the measurement of the pH were based on technical reasons related to the routine of the dental office, determined by studies in the literature8,24,25.
The methodology used is valid once the hydroxyl ions on the external root surface are immediately noted by the electrode of the pH meter. Some studies21,29 were carried with similar methodology. The results of this present study differ from those of the literature6,21, which demonstrated that there was not pH alteration at periods shorter than 14 days with use of viscous vehicles, such as Calen paste and chlorhexidine gel. The dissociation of calcium hydroxide is directly proportional to the vehicles used, because the aqueous vehicles enable to reach a pH close to 12.6, due to a faster dissociation and diffusion velocity of the hydroxyl ions. Because the viscous vehicles exhibit smaller dissociation and diffusion velocity of hydroxyl ions they require longer times to reach high pH thresholds3.
Concerning to the seven groups of the study, each one with a different intracanal medication, they were tested as aforementioned described. The sealing of the apical foramen was carried out to avoid to the direct contact of the medication with the solution (saline) in which the specimens were immersed, therefore avoiding influences on the results. This occurs because which causes the pH alteration of the samples would be, according to the literature, the diffusion of the intracanal medication through the dentinal tubules of the dentinal tissue, reaching the periodontal tissues consequently with pH increase of the external root surface.
It is necessary to emphasize that for the medication diffusion through dentinal tubules to occur and consequently alter the pH of the external environment, the tubules should be the most preserved as possible and this was obtained with the maximum conservation of the teeth through their washing with saline just after the extraction and storage in 10% formalin solution.
The results of the pH measurements revealed that the most beneficial association to increase the pH progressively over time was calcium hydroxide with 2% chlorhexidine gel, regardless of the use of EDTA after the ending of the instrumentation procedures of the root canals.
The use of EDTA prior to the application of the intracanal medication has a chelating function of the removal of the smear layer, opening of the dentinal tubules and facilitation of the diffusion of the intracanal medication inside the canals5,6,8,18. However, the results of this present study demonstrated there was no influence of EDTA on the process of the hydroxyl ion diffusion through the dentinal tissue.
Although the Calen® paste is for many dentists more practical for usage and demonstrated adequate initial results it did not show the maintenance of the pH increasing, as expected with pastes with viscous vehicles. Probably, the fact of the paste is ready for use, i. e., it had been mixed long time ago, the calcium and hydroxyl ions react internally and promote the formation of calcium carbonate, which is stable.
There is no ideal intracanal medication, that is, one that is able to influence directly on the clinical signs and symptoms of the patients. Notwithstanding, the researches must be conducted to guide the best choices of the existing materials as well as their associations. The association of biocompatible substances with high antimicrobial capacity directs the ideal intracanal medication because the endodontic treatment aims to eliminate of the aggressive agent within the root canals and to promote its tridimensional sealing. Further studies analyzing the profile of the medicaments over time and which verify their antimicrobial activities and formation of compounds should be executed with the goal of searching the ideal substances and associations for dental and endodontic purposes.
Conclusion
According to the results obtained, it can be concluded that:
• All calcium hydroxide pastes used demonstrated the capacity of releasing hydroxyl ions at the initial period of use, with pH increasing;
• The use of 2% chlorhexidine gel provided the gradual pH increasing over time;
• The removal of the smear layer did not influence on the pH of the root surface of teeth with canals filled with calcium hydroxide paste.
References
1. Atila-Pektas B, Yurdakul P, Gülmez D, Görduysus Ö. Antimicrobial effects os root canal medicaments against Enterococcus faecalis and Streptococcus mutans. Int Endod J. 2012;45(12):1-6. [ Links ]
2. Barekatain B, Hasheminia SM, Shadmehr E, Attary Z. The effect of calcium hydroxide placement on ph and calcium concentration in periapical environment: an in vitro study. Indian J Dent Res. 2012;23(2):226-9. [ Links ]
3. Barreto SS, Luisi SB, Fachin EVF. Importância da dissociação dos ions cálcio e hidroxila de pastas de hidróxido de cálcio. Rev Clín Pesq Odontol. 2005;1(4):37-46. [ Links ]
4. Duarte MAH, Balan NV, Zeferino MA, Vivan RR, Morais CAH, Tanomaru-Filho M et al. Effect of ultrasonic activaction on pH and calcium released by calcium hydroxide pastes in simulated external root resorption. JOE. 2012;38(6):834-7. [ Links ]
5. Ercan E, Dalli M, Dülgergil ÇT, Yaman F. Effect of intracanal medication with calcium hydroxide and 1% chlorhexidine in endodontic retreatment cases with periapical lesions: an in vivo study. J Formos Med Assoc. 2007;106(3):217-24. [ Links ]
6. Estrela C, Pécora JD, Souza Neto MD, Estrela CRA, Bammann LL. Effect of vehicle on antimicrobial properties of calcium hydroxide pastes. Braz Dent J. 1999;10:63-72. [ Links ]
7. Farhad AR, Barekatain B, Allameh M, Narimani T. Evaluation of the antibacterial effect of calcium hydroxide in combination with three different vehicles: an in vitro study. Dent Res J. 2012;9(2):167-72. [ Links ]
8. Ferraz CCR, Gomes BPFA, Zaia AA, Souza-Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001;27:452-5. [ Links ]
9. Gomes BPFA, Lilley JD, Drucker DB. Associations of endodontic symptoms and signs with particular combinations of specific bacteria. Int Endod J. 1996;29(2):69-75. [ Links ]
10. Kazemiroor M, Tabrizizadeh M, Dastani M, Hakimian R. The effect of retreatment procedure on the changes at the surface of root dentin using two different calcium hydroxide pastes. J Conserv Dent. 2012;15(4):346-50. [ Links ]
11. Kontakiotis E, Nakou M, Georgopoulou M. In vitro study of the indirect action of calcium hydroxide on the anaerobic flora of the root canal. Int Endod J. 1995;28(6):285-9. [ Links ]
12. Lee J-K, Baik JE, Yun C-H, Lee K, Han SH, Lee W et al. Chlorhexidine gluconate attenuates the ability of lipoteichoic acid from Enterococcus faecalis to stimulate toll-like receptor 2. J Endod. 2009;35(2):212-5. [ Links ]
13. Maniglia-Ferreira C, Bonifácio KC, Fröner IC, Ito IY. Evaluation of the antimicrobial activity of three irrigating solutions in teeth with pulpal necrosis. Braz Dent J. 1999;10:15-27. [ Links ]
14. Maniglia-Ferreira C, Rosa OPS, Torres SA, Ferreira FBA, Bernardineli N. Activity of endodontic antibacterial agents against selected anaerobic bacteria. Braz Dent J. 2002;13(2):118-22. [ Links ]
15. Mohammadi Z, Shalavi S. Is clorhexidine an ideal vehicle for calcium hydroxide? A microbiologic review. Iran Endod J. 2012;7(3):115-22. [ Links ]
16. Montero JC, Mori GG. Assessment of ion diffusion from a calcium hydroxide-propolis paste through dentin. Braz Oral Res. 2012;26(4):318-22. [ Links ]
17. Mori GG, Ferreira FC, Batista FRS, Godoy AMS, Nunes DC. Evaluation of the diffusion capacity of calcium hydroxide pastes through the dentinal tubules. Braz Oral Res. 2009;23(2):113-8. [ Links ]
18. Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressing of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142-9. [ Links ]
19. Pacios MG, de La Casa ML, Bulacio M de los A, Lopez ME. Influence of different vehicles on the pH of calcium hydroxide pastes. J Oral Sci. 2004;46(2):107-11. [ Links ]
20. Pinheiro ET, Gomes BPFA, Ferraz CCR, Sousa ELR, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36(1)1-11. [ Links ]
21. Repeke HP, Westphalen VPD, Silva Neto UX, Carneiro E, Fariniuk LF, Sousa MH et al. Estudo do pH de três diferentes pastas de hidróxido de cálcio. Rev Clín Pesq Odontol. 2008;4(3):169-73. [ Links ]
22. Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994;20(3):127-9. [ Links ]
23. Siqueira Jr JF, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;23:361-9. [ Links ]
24. Siqueira Jr JF, Uzeda M. Intracanal medicaments: evaluation of the antimicrobial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J Endod. 1997;23:167-9. [ Links ]
25. Sirén EK, Haapasalo MPP, Waltimo TMT. In vitro antibacterial effect of calcium hydroxide combined with chlorhexidine or iodine potassium iodine on Enterococcus faecalis. Eur J Oral Sci. 2004;112:326-31. [ Links ]
26. Sjögren U, Figdor D, Persson S. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297-306. [ Links ]
27. Sundqvist G, Figdor D, Persson S. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86-93. [ Links ]
28. Torabinejad M, Ung B, Kettering JD. In vitro bacterial penetration of coronally unsealed endodontic treated teeth. J Endod. 1990;16:566-9. [ Links ]
29. Tronstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I. pH changes in dental tissue after root canal filling with calcium hydroxide. J Endod. 1981;7(1):17-21. [ Links ]
30. Vianna ME, Gomes BP, Sena NT, Zaia AA, Ferraz CC, Souza Filho FJ. In vitro evaluation of the susceptibility of endodontic pathogens to calcium hydroxide combined with different vehicles. Braz Dent J. 2005;16:175-80. [ Links ]
31. Vianna ME, Horz HP, Gomes BPFA, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39:484-92. [ Links ]
32. Yücel AC, Aksoy A, Ertas E, Güvenc D. The pH changes of calcium hydroxide mixed with six different vehicles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(5):712-7. [ Links ]
 Corresponding author:
Corresponding author:
Cláudio Maniglia-Ferreira
Rua Bento Albuquerque, n. 685, apto. 1.102
CEP 60192-060 – Fortaleza – CE – Brasil
E-mail: maniglia@unifor.br
Received for publication: November 28, 2012
Accepted for publication: December 17, 2012













