Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.2 Joinville Abr./Jun. 2013
ORIGINAL RESEARCH ARTICLE
In vivo evaluation of tissue response to new endodontic sealers
Annecy Geib da SilvaI; Nádia Carolina Teixeira MarquesII; Natalino Lourenço NetoII; Tamara Zeponi Fernandes de MeloII; Vivian Agostino Biella PassosI; Cleide Felício Carvalho CarraraI; Thais Marchini OliveiraII
I Sector of Pediatric Dentistry, Hospital of Rehabilitation of Craniofacial Anomalies of Bauru, University of São Paulo – Bauru – SP – Brazil
II Department of Pediatric Dentistry, Orthodontics and Collective Health, Discipline of Pediatric Dentistry, School of Dentistry of Bauru, University of São Paulo – Bauru – SP – Brazil
ABSTRACT
Introduction: The sealers can be in direct contact with the periapical tissues. Thus, these materials must have appropriate physical and biological properties, providing conditions for repair to occur.
Objective: The aim of this study was to evaluate the response of rat subcutaneous tissue to endodontics sealers.
Material and methods: Three materials comprised the groups: group I – Zinc Oxide, Eugenol and Iodoform paste, group II – Portland cement with propylene glycol, and group III – MTA Fillapex® (Angelus). These materials were placed in polyethylene tubes and implanted into dorsal connective tissue of Wistar rats for seven and 15 days. The specimens were stained with hematoxylin and eosin and evaluated regarding to inflammatory reaction parameters through a light microscope. The data were compared using Kruskal-Wallis test with significance level of 5%. The intensity of inflammatory response against the sealers was analyzed by two blinded and previously calibrated observers for all experimental periods.
Results: The histological evaluation showed that all the materials caused a moderated inflammatory reaction at seven days which decreased with time. A greater inflammatory reaction was observed at seven days in group I. The other specimens had significantly less inflammatory cells when compared to this group. Tubes with MTA Fillapex® presented some giant cells, macrophages and lymphocytes after seven days. At 15 days, the presence of fibroblasts and collagen fibers was observed indicating normal tissue healing. The group II showed similar results to those observed in MTA Fillapex® already at seven days. At 15 days the inflammatory reaction presented was almost absent at the tissue, with many collagen fibers indicating normal tissue healing. Statistical analysis showed a significant statistical difference amongst the group I (seven days) and II (15 days) (p < 0.05). In the other groups no significant statistical differences were observed.
Conclusion: MTA Fillapex® and Portland cement with propylene glycol were more biocompatible than the other tested cements.
Keywords: biocompatibility; tissue response; root filling materials; Portland cement; MTA.
Introduction
The endodontic cements can be in direct contact with the periapical tissues. Thus, these materials should present physical and biological properties, providing conditions for the occurrence of the repair 9,10,18,19. For this purpose, it is very important to know the biological compatibility of the sealers because if they were very irritating to the periapical tissues, all endodontic treatment will be damaged and, consequently, the capacity of repairing of the periapical region will undergo significant interferences 10. Considering the importance of knowing the compatibility of these materials, several studies using implants on conjunctive tissues of rats have been carried out aiming to evaluate the biological characteristics of the endodontic sealers 6-8,10,12,18,23,26,28-30.
For deciduous teeth, in addition to the compatibility of the sealers employed, one should considers the use of resorbable sealers, i.e., which can be phagocytized by the cells of the tissues of the periapical area; also, the deciduous teeth undergo the physiologic root resorption and at this phase they can be in direct contact with the permanent tooth bud, therefore it is fundamental that they do not cause any alteration in this process 1,24.
The mineral trioxide aggregate (MTA) sealer has been the subject of a series of experimental studies analyzing both their physical-chemical and biological properties with good clinical and laboratorial results 2,4,11,14,16,20,22,25,29. Until recently, the MTA does not exhibited an adequate formulation to be used as endodontic sealer; however, a new MTA formulation so-called MTA Fillapex® (Angelus®) has been indicated to be utilized as endodontic sealer in root canal filling. Because it has a chemical composition similar to that of the MTA cement, a similar biological response is also expected. Another material receiving attention in the scientific literature is the Portland cement, because it exhibits the composition and performance similar to MTA. It also enables a neoformation of the mineralized tissue and the maintenance of the vitality of the pulpal remnant 2,3,15,17.
Aiming to assess other therapeutic options for endodontic filling of the root canals of deciduous teeth, the aim of this study was to evaluate the tissue response of endodontic sealers on the subcutaneous tissue of rats.
Material and methods
This present study was approved by the Ethical Committee in Animal Research of the School of Dentistry of Bauru (University of São Paulo), under process number. 025/2010.
Selection of the animals and surgery
Twelve Wistar male rats, weighing between 200 and 250 g (approximately 60 days), coming from the Central Vivarium of the School of Dentistry of Bauru (University of São Paulo). On the dorsum of each animal, three tubes containing each materialwas introduced and divided as follows: GI – zinc oxide (80%) + eugenol + iodoform (20%); GII – Portland cement + propylene glycol; GIII – MTA Fillapex (Angelus®). It was opted to use the lateral side of the polyethylene tube as negative control of the inflammation.
The animals were anesthetized with ketamine hydrochloride and xylazine hydrochloride (Dopalen® – 0.4 ml/kg and Anasedan® – 0.02 ml/kg, intramuscular route). Following, the hand trichotomy on the dorsal region of the animal and the antisepsis with gauze embedded in 5% iodinated alcohol were executed. Next, two longitudinal incisions on the dorsum of the animal, one anterior e another posterior were carried out. After the divulsion of the tissues with the aid of blunt scissors, the polyethylene tubes were implanted on the dorsum of the animal with the aid of a surgical trochar. In the anterior region, one tube was implanted at each side of the animal, with distance of 5 cm between each other and one tube was inserted at the posterior area. The materials of groups I and II were mixed onto glass plates, comprising: the measuring spoon of the MTA kit (Angelus®), and two drops of eugenol for group I and two drops of propylene glycol for group II, obtaining a final consistence of paste. The MTA Fillapex® was used directly from its package as recommended by its manufacturer. All sealers were introduced into the polyethylene tubes with 10 mm of lenght, 1.5 mm of internal diameter and 2 mm of external diameter. The tubes were initially kept for 1 hour submersed in 1% sodium hypochlorite and then were washed with saline solution and dried with sterile gauze. The tubes were filled with the material following the aforementioned division of the groups. The samples were left in the dorsum of the animal for seven and 15 days. After these experimental periods, the animals were killed with the aid of an excessive dose of anesthetic drug to obtain the cuts for histological analysis.
Histological analysis
The pieces containing the implants were removed and fixed in 10% formalin and kept for 48 hours in individual and identified flasks. Elapsed the period of 48 hours for fixation, the pieces were washed in 70% alcohol and the tubes were carefully removed. Next, the pieces were dehydrated in consecutive baths with alcohol at increasing concentrations (70%, 80%, 90% and absolute), diaphanized with xylol and included in paraffin to obtain blocks.
Then, the serial cuts were processed with 5 μm of thickness in a microtome (Leica RM 2165); next, the material cut was distended and put in laminas which were kept in an incubator at 60ºC for deparaffinization. The laminas were immersed in Harris' hematoxylin and washed in running water to remove the dye excess. After the removal of the water excess, the cuts were immersed in eosin for five minutes, dehydrated, clarified and the laminas were mounted to the reading in optical microscope (Olympus CH-2).
The histological evaluation was double blinded. It was verified the intensity of the inflammatory infiltrate and repair tissue. The materials were histologically analyzed and the tissue and cellular morphology was described regarding to the experimental period.
Results
The evaluation of the histological events was performed by two previously calibrated examiners (Kappa = 0.96), through optical microscope, at x10 to x40 magnification, taking into consideration the following histopathological events inflammatory infiltrate, cellularity, vascularization and macrophagic activity in the reactional capsule.
Group I – zinc oxide, eugenol and iodoform (ZOE + iodoform)
At seven days, an intense mononuclear inflammatory infiltrate (lymphocytes and plasmocytes), macrophages and giant cells were seen. Great amount of connective tissue and congested blood vessels. The presence of the remnant material was constant in all laminas observed. Conjunctive tissue in repairing process with considerable presence of fibroblasts on the region inflamed (figures 1.1 and 1.2). In one of the laminas observed, it was noted areas of tissue necrosis with presence of polymorphonuclear neutrophils. At 15 days, it could be still noted the constant presence of material, indicating its difficult resorption, associated with a mononuclear inflammatory infiltrate, congested blood vessels and disorganized conjunctive tissue (figures 2.1 and 2.2).
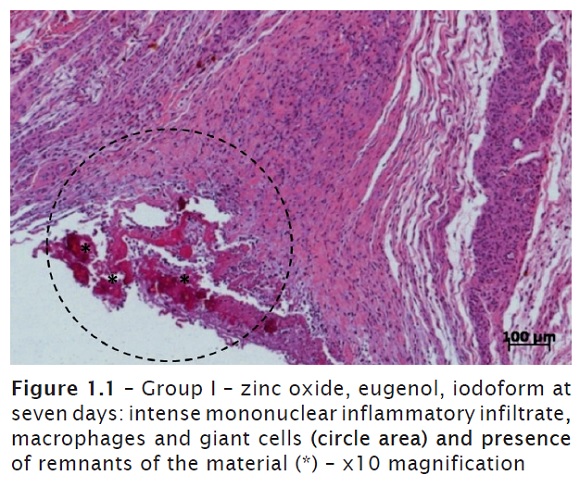
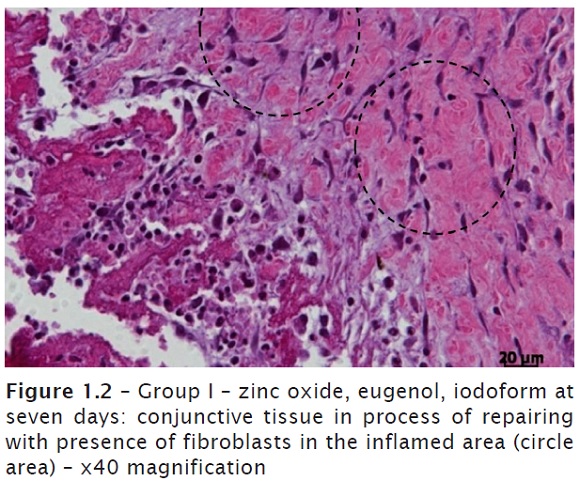
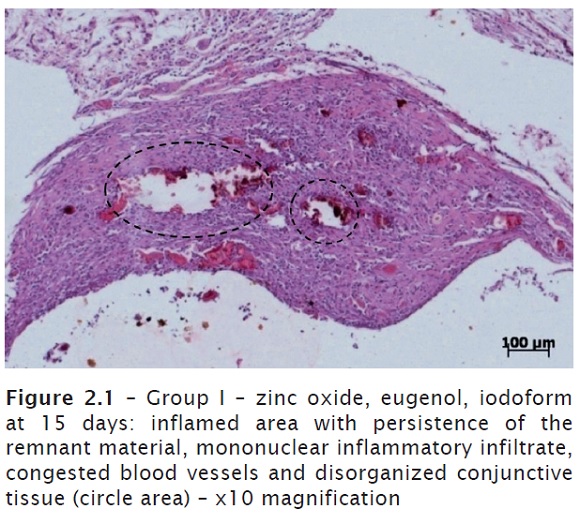
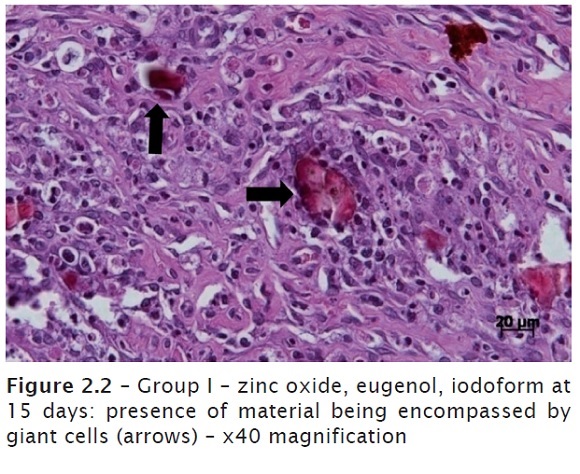
Group II – Portland + propylene glycol
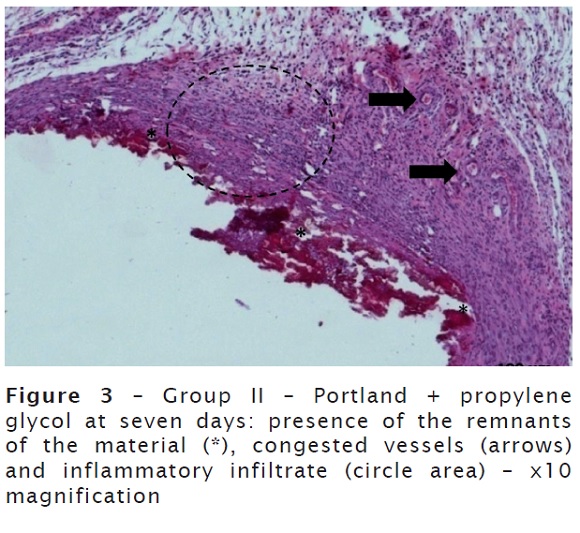
At seven days, there was a great amount of material, discreet mononuclear inflammatory infiltrate, giant cells and congested blood vessels. Conjunctive tissue in formation, still very immature on the inflamed area of the opening of the tube (figure 3).
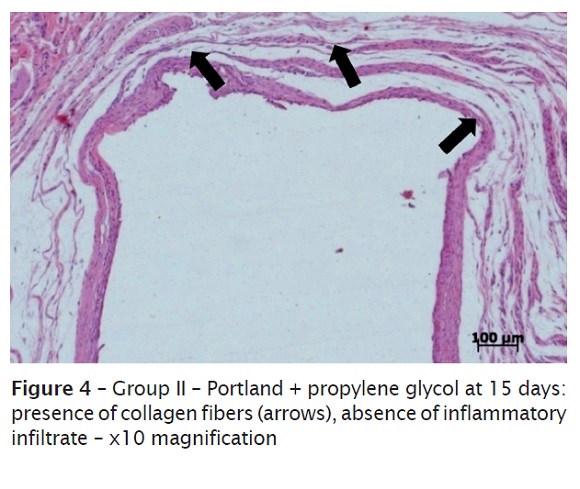
At 15 days, there was the predominance of mature fibrous conjunctive tissue, exhibiting greater amount of collagen fibers and fewer cells in the areas of repairing (figure 4). Absent or discreet inflammatory infiltrate were observed, and when present they were involving the remnants of the material (foreign body-like multinucleated giant cells).
Group III – MTA Fillapex®
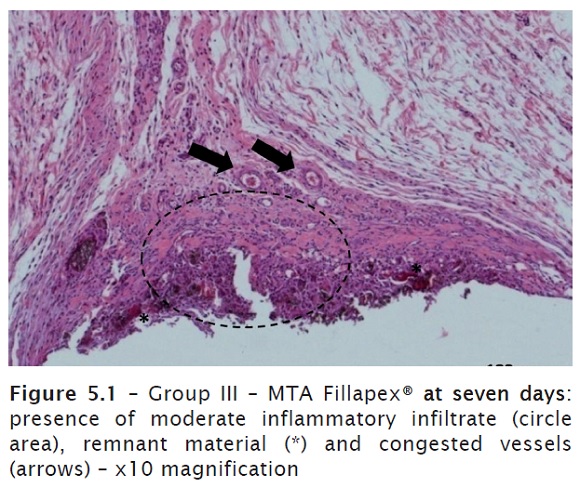
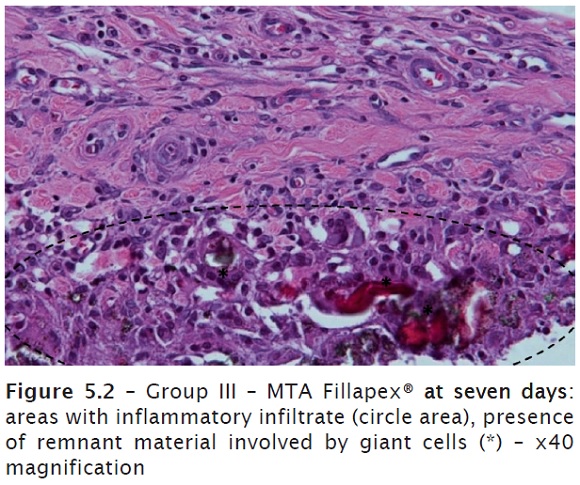
At seven days, a fibrous conjunctive tissue was observed with moderate, predominantly mononuclear inflammatory infiltrate and presence of multinucleated giant cells, especially close to the remnants of the material. Presence of congested blood cells. Formation of connective tissue close to the opening of the tube (figures 5.1 and 5.2).
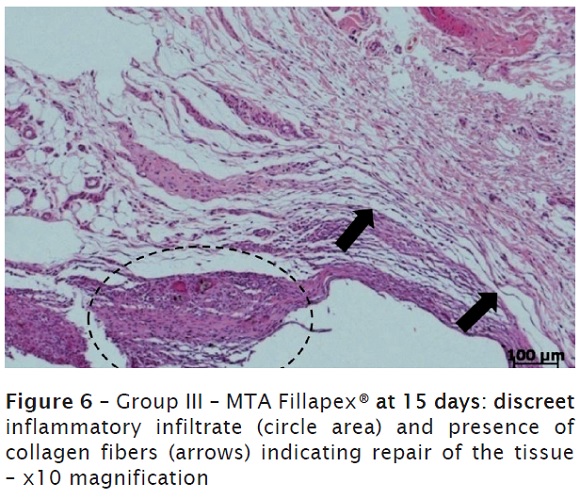
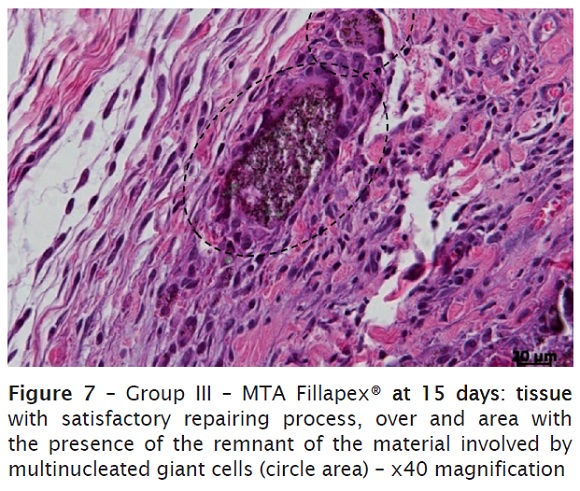
At 15 days, it was verified a connective tissue richly cellularized, exhibiting discreet mononuclear inflammatory infiltrate and blood vessels, some congested (figure 6). It was still noted the remnant material involved by multinucleated giant cells (figure 7).
Discussion
It is recognized that the endodontic sealers can be very irritating to the tissues of the periapical region, cause inflammation or promote the tissue necrosis in this area 8,10. The methodology employed with polyethylene tubes for the study of endodontic sealers is a tool demonstrably satisfactory for biocompatibility tests, once it mimics the root canal conditions because the ending of the tube containing the material is in contact with the conjunctive tissue, representing the apical foramen/apical tissue relationship 3,9,10. Concerning to the evaluation time, there is not a consensus among the authors in the current literature. The study period was based on the studies of Gomes-Filho et al. 9, Gomes-Filho et al. 10 and Mori et al. 17. According with the literature, the lateral side of the polyethylene tube was used as negative control, once it was not in contact with the materials tested, therefore resulting in the histological analysis of the direct contact of the tissue with the material of the tube 30.
In studies involving the conjunctive tissue, the biocompatibility is demonstrated by the formation of the fibrous capsule surrounding the tubes implanted, indicating the repairing activity and suggesting the good acceptance of the material by the tissues 3,4,12,15,27. All sealers implanted provoked a tissue reaction characterized by a chronic inflammatory response from mild to moderate, fibroangioblastic proliferation formation of collagen fibers, evidencing the biocompatibility of the materials over time 6,23,28,30. Although these tests are not even ideal, the data obtained is of great value for the evaluation of new materials that are launched into the market 4,21.
The group of ZOE + iodoform was chosen because this material is largely employed in Endodontics of deciduous teeth and iodoform is a radiopacifying agent added to ZOE 1. This material exhibited a biological behavior less favorable than that of the other groups and an inflammatory infiltrate at the initial periods that persisted during all the period evaluated. In the analyses of 15 days, it was still observed great amounts of the remnants of the material in the tissue, in addition to a discreet formation of collagen fibers 8-10.
The morphological analysis of the laminas obtained from the materials tested demonstrated that the biological behavior of Portland cement and MTA Fillapex® sealer was similar at almost periods evaluated. Both sealers induced a discreet inflammatory infiltrate at the initial periods (seven days), which decreased over time, and significant fibroblastic and collagen fiber proliferation at the final periods (15 days). Such findings corroborate with several studies which demonstrated the similarities in the basic chemical composition, mechanism of action and cytotoxicity of both materials tested, differing only in the addition of some resin and radiopacifying components in the MTA Fillapex® formula 3,9,15,18,19,25,31.
Aiming to reach a greater similarity between the sealers tested, the propylene glycol was added to the Portland cement, once such viscous vehicle is still little studied in association with this cement but it results in a better flowing and longer setting time, without compromising its physical-chemical properties and consequently giving in this present study characteristics very similar to those showed by MTA Fillapex® 5.
Despite the similarity between Portland cement and MTA, the latter can in some cases show more favorable results because it is fabricated under controlled conditions free of impurities. The Portland cement can contain some impurities coming from the mineral from which it is extracted 2,11,16,20,25,30. Other authors demonstrated in their studies that MTA can exhibit a less favorable result with greater occurrence of inflammation in the initial analyses because of its high pH and temperature at the initial moments of its setting, which does not occur with the Portland cement 2,13. Such fact is in agreement with the results of this study, justifying the data found.
Conclusion
The Portland cement and MTA Fillapex® exhibited the best biological behavior when the provoked inflammatory intensity and the tissue repairing evolution was evaluated; however, further studies are necessary for the most accurate indication of these materials.
References
1. Barcelos R, Santos MP, Luiz RR, Maia LC. ZOE paste pulpectomies outcome in primary teeth: a systematic review. J Clin Pediatr Dent. 2011;35(3):241-8. [ Links ]
2. Conti TR, Sakai VT, Fornetti AC, Moretti ABS, Oliveira TM, Lourenço Neto N et al. Pulpotomies with portland cement in human primary molars. J Appl Oral Sci. 2009;17(1):66-9. [ Links ]
3. Coutinho-Filho T, De-Deus G, Klein L, Manera G, Peixoto C, Gurgel-Filho ED. Radiopacity and histological assessment of Portland cement plus bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Dec;106(6):69-77. [ Links ]
4. Danesh F, Tootian Z, Jahanbani J, Rabiee M, Fazelipour S, Taghva O et al. Biocompatibility and mineralization activity of fresh or set white mineral trioxide aggregate, biomimetic carbonated apatite, and synthetic hydroxyapatite. J Endod. 2010 Jun;36(6):1036-41. [ Links ]
5. Duarte MA, Alves de Aguiar K, Zeferino MA, Vivan RR, Ordinola-Zapata R, Tanomaru-Filho M et al. Evaluation of the propylene glycol association on some physical and chemical properties of mineral trioxide aggregate. Int Endod J. 2012 Jun;45(6):565-70. [ Links ]
6. Garcia LFR, Lia RCC, Lopes RA, Oliveira DA, Pires-De-Souza FCP, Santos HSL. Análise morfológica e morfométrica do tecido subcutâneo de ratos submetidos à ação de pasta de hidróxido de cálcio e óleo de Ricinus communis. Ciênc Odontol Bras. 2008 Jul/Sep;11(3):47-54. [ Links ]
7. Gencoglu N, Sener G, Omurtag GZ, Tozan A, Uslu B, Arbak S et al. Comparision of biocompatibility and cytotoxicity of two new root canal sealers. Acta Histochem. 2010 Nov;112(6):567-75. [ Links ]
8. Ghanaati S, Willershausen I, Barbeck M, Unger RE, Joergens M, Sader RA et al. Tissue reaction to sealing materials: different view at biocompatibility. Eur J Med Res. 2010 Nov;15(11):483-92. [ Links ]
9. Gomes-Filho JE, Watanabe S, Lodi CS, Cintra LT, Nery MJ, Filho JÁ et al. Rat tissue reaction to MTA Fillapex®. Dent Traumatol. 2012 Dec;28(6):452-6. [ Links ]
10. Gomes-Filho JE, Gomes BP, Zaia AA, Ferraz CR, Souza-Filho FJ. Evaluation of the biocompatibility of root canal sealers using subcutaneous implants. J Appl Oral Sci. 2007 Jun;15(3):186-94. [ Links ]
11. Holland R, de Souza V, Nery MJ, Faraco Júnior IM, Bernabé PF, Otoboni Filho JA et al. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, Portland cement or calcium hydroxide. Braz Dent J. 2001;12(1):3-8. [ Links ]
12. Khashaba RM, Moussa MM, Chutkan NB, Borke JL. The response of subcutaneous connective tissue to newly developed calcium phosphate-based root canal sealers. Int Endod J. 2011 Apr;44(4):342-52. [ Links ]
13. Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human odontoblasts. J Biomed Mater Res. 1997;37(3):432-9. [ Links ]
14. Lotfi M, Vosoughhosseini S, Saghiri MA, Mesgariabbasi M, Ranjkesh B. Effect of white mineral trioxide aggregate mixed with disodium hydrogen phosphate on inflammatory cells. J Endod. 2009 May;35(5):703-5. [ Links ]
15. Martínez Lalis R, Esaín ML, Kokubu GA, Willis J, Chaves C, Grana DR. Rat subcutaneous tissue response to modified Portland cement, a new mineral trioxide aggregate. Braz Dent J. 2009;20(2):112-7. [ Links ]
16. Moretti AB, Sakai VT, Oliveira TM, Fornetti AP, Santos CF, Machado MA et al. Effectiveness of mineral trioxide aggregate, calcium hydroxide and formocresol for pulpotomies in primary teeth. Int Endod J. 2008;40(8):653-60. [ Links ]
17. Mori GG, Moraes IG, Nunes DC, Castilho LR, Poi W, Capaldi ML. Biocompatibility evaluation of tendrovate paste in rat's subcutaneous tissue. Dent. Traumatol. 2009;25(2):209-12. [ Links ]
18. Nassri MRG, Lia RCC, Bombana AC. Análise da resposta tecidual de dois cimentos endodônticos. J Appl Oral Sci. 2003;11(1):9-14. [ Links ]
19. Okino Neto K, Moura AAM, Davidowicz H. Estudo comparativo da reação tecidual conjuntiva de ratos, frente a três cimentos endodônticos resinosos. J Health Sci Inst. 2010;28(1):67-70. [ Links ]
20. Oliveira TM, Sakai VT, Silva TC, Santos CF, Machado MA, Abdo RC. Repair of furcal perforation treated with mineral trioxide aggregate in a primary molar tooth: 20-month follow-up. J Dent Child. 2008 May-Aug;75(2):188-91. [ Links ]
21. Parirokh M, Mirsoltani B, Raoof M, Trabrizchi H, Haghdoost AA. Comparative study of subcutaneous tissue response novel root-end filling material and white and gray mineral trioxide aggregate. Int Endod J. 2011;44(4):283-9. [ Links ]
22. Peng L, Ye L, Tan H, Zhou X. Evaluation of the formocresol versus mineral trioxide aggregate primary molar pulpotomy: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(6):40-4. [ Links ]
23. Perassi FT, Pappen FG, Bonetti Filho I, Leonardo RT, Ykeda F, Ramalho LTO. Estudo morfológico da resposta tecidual a quatro cimentos endodônticos. Rev Odont UNESP. 2008;37(2):117-24. [ Links ]
24. Queiroz AM, Assed S, Consolaro A, Nelson-Filho P, Leonardo MR, Silva RA et al. Subcutaneous connective tissue response to primary root canal filling materials. Braz Dent J. 2011;22(3):203-11. [ Links ]
25. Sakai VT, Moretti AB, Oliveira TM, Fornetti AP, Santos CF, Machado MA et al. Pulpotomy of human primary molars with MTA and Portland cement: a randomised controlled trial. Br Dent J. 2009 Aug 8;207(3):E5; discussion 128-9. [ Links ]
26. Scarparo RK, Grecca FS, Fachin EV. Analysis of tissue reactions to methacrylate resin-based, epoxy resin-based, and zinc oxide-eugenol endodontic sealers. J Endod. 2009 Feb;35(2):229-32. [ Links ]
27. Scarparo RK, Haddad D, Acasigua GA, Fossati AC, Fachin EU, Grecca FS. Mineral trioxide aggregate-based sealer: analysis of tissue reactions to a new endodontic material. J Endod. 2010;36(7):1174-8. [ Links ]
28. Silva-Herzog D, Ramírez T, Mora J, Pozos AJ, Silva LA, Silva RA et al. Preliminary study of the inflammatory response to subcutaneous implantation of three root canal sealers. Int Endod J. 2011 May;44(5):440-6. [ Links ]
29. Sumer M, Muglali M, Bodrumlu E, Guvenc T. Reactions of connective tissue to amalgam, intermediate restorative material, mineral trioxide aggregate, and mineral trioxide aggregate mixed with chlorhexidine. J Endod. 2006 Nov;32(11):1094-6. [ Links ]
30. Zafalon EJ, Versiani MA, de Souza CJ, Moura CC, Dechichi P. In vivo comparison of the biocompatibility of two root canal sealers implanted into the subcutaneous connective tissue of rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 May;103(5):88-9. [ Links ]
31. Zmener O, Martinez Lalis R, Pameijer CH, Chaves C, Kokubu G, Grana D. Reaction of rat subcutaneous connective tissue to a mineral trioxide aggregate-based and a zinc oxide and eugenol sealer. J Endod. 2012 Sep;38(9):1233-8. [ Links ]
 Corresponding author:
Corresponding author:
Thais Marchini Oliveira
Faculdade de Odontologia de Bauru – Universidade de São Paulo
Departamento de Odontopediatria, Ortodontia e Saúde Coletiva
Alameda Dr. Octávio Pinheiro Brisolla, 9-75
CEP 17012-901 – Bauru – SP – Brasil
E-mail: marchini@usp.br
Received for publication: October 22, 2012
Accepted for publication: December 18, 2012













