Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.2 Joinville Abr./Jun. 2013
ORIGINAL RESEARCH ARTICLE
Staining susceptibility of methacrylate and silorane-based materials: influence of resin type and storage time
Leonardo Fernandes da CunhaI; Lino Oliveira Carvalho de SantanaII; Samantha Schaffer Pugsley BarattoII; José MondelliI; Gisele Aihara HaragushikuII; Carla Castiglia GonzagaII; Adilson Yoshio FuruseII
I Department of Operative Dentistry, Endodontics and Dental Materials, Bauru School of Dentistry – Bauru – São Paulo – Brazil.
II Master Student of the Science Program in Clinical Dentistry, Positivo University – Curitiba – PR – Brazil.
ABSTRACT
Introduction: The color stability of composite resins is a fundamental factor in their clinical behavior.
Objective: To evaluate the color stability of composite resins of different colors exposed to a cola-based soft drink after different storage periods. Additionally, three methacrylate-based materials and one silorane-based material were evaluated.
Material and methods: Specimens of three methacrylate-based materials (Opallis EA3, DA3 and T-Neutral; Filtek Supreme XT A3E, A3D and CT; 4 Seasons A3 Enamel, A3 Dentin and High Value) and one silorane-based material (Filtek P90 A3) were prepared, light-cured for 40 s, and manually polished with Sof-Lex discs. Samples were stored for 1 h, 24 h or 7 days. The color was evaluated by CIE-Lab system before and after immersion for 10 min in a cola-based soft drink. Color variation (ΔE) was calculated from individual values of L*, a* and b*, being considered imperceptible when < 1, clinically acceptable when ≤ 3.3, and clinically inacceptable when higher than 3.3. Data were evaluated by two-way Anova and Dunnett's T3 tests (α = 0.05).
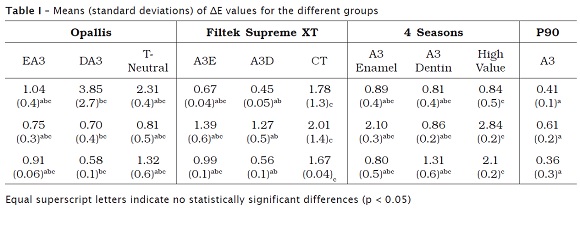
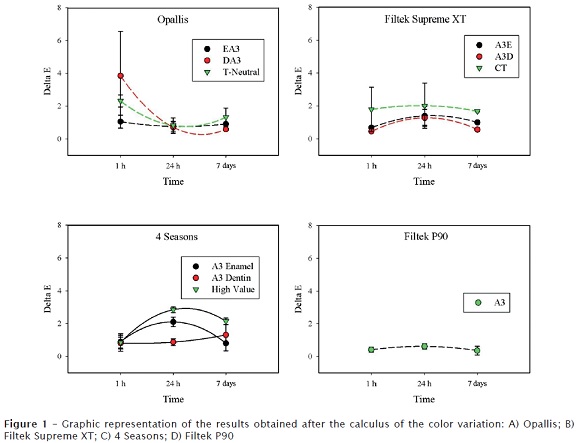
Results: There were differences among the resins (p < 0.001), with an interaction effect being also observed (p < 0.001). Storage time was not significant (p = 0.246). P90 showed a ΔE smaller than one unit at all studied times. Supreme XT CT and 4 Seasons High Value showed higher ΔE, but not above the critical value of 3.3. The only material that showed ΔE higher than 3.3 was Opallis DA3 after 1 h of storage.
Conclusion: The silorane-based composite resin showed smaller ΔE at the times studied.
Keywords: resin composites; color; pigmentation.
Introduction
The resin composites have been largely accepted as restorative material in Dentistry. Since its appearance, additionally to the use of many monomer types, several types and sizes of filler particles have been tested. These particles are incorporated to increase their physical and chemical properties 7. Despite of the greatest use in Dentistry, it is known to date that most of the composites are based on methacrylates as Bis-GMA, Bis-EMA, UDMA and TEG-DMA 15,20.
With the advancement of the composites, the use of these materials started to increase due to the improvement of the physical-chemical and aesthetical properties. Thus, the requirements regarding to the perfect matching between the color of the restorations and teeth became even greater, and, one of the most common reasons to the replacement of the aesthetical restorations is the incorporation of pigments which compromise the aesthetical harmony of the smile 8,30.
The aesthetical properties are directly related with the degree of the opacity, translucency and opalescent effects. At the long term, factors as color stability, roughness, retention, and surface brightness can determine the success or failure of the restoration 3,12.
The pigmentation of the resin composite restorations can be caused by several intrinsic and extrinsic factors. Intrinsic factors are those involving the discoloration of the resin itself related to the alterations in the resin matrix and in the interface of the matrix. The intrinsic discoloration can be also attributed to the insufficient polymerization, immersion in liquid solution for longer periods, quality of both the light-curing and the polymers. On the other hand, extrinsic alterations can be attributed to external events, among them, it is the insufficient degree of polymerization, water sorption, heating, and adsorption of food dyes, such as coffee, tea, and red wine 3,6,11,17.
With the aim of decreasing the polymerization shrinkage and sorption of pigments, silorane-based resin composites have been developed 14. As advantages, silorane-based resin composites show low polymerization shrinkage, low stress and good insolubility in simulators of biological fluids 9. Additionally, a good color stability in artificial aging tests in which the material is exposed to high doses of ultraviolet light have been reported 12.
It is known that the polymerization process of resin-based materials is continue and can be continued for longer periods, even when light-cured resins are considered 13. Because of this continued resin and because resin composites tend to absorb water at the first hours after its polymerization, it is common to recommend that the patient avoids the consumptions of dyes at the first hours or days. Therefore, the exposure to dyes coming from daily food intake needs to be evaluated.
The dyes are present in most of the foods. Beverages, as cola-based soft drinks, black tea, coffee, red wine, and fruit juices seem to produce a greater influence on the color of both the teeth 31 and resin composites 29. It should be highlighted that the consumption of these beverages is highly popular and the inhibition of its ingestion for aesthetical purposes is impracticable to most of the population. This concern has been the reason of studies on other modalities of aesthetical treatments, such a dental bleaching 28.
The aim of this study was to evaluate the color stability of resin composites of different colors exposed to a cola-based soft drink after different time periods. Additionally, three methacrylate-based and one silorane-based material were evaluated.
Material and methods
Specimens of three methacrylate-based materials (Opallis EA3, DA3 e T-Neutral, FGM, Joinville, Brazil; Filtek Supreme XT A3E, A3D and CT 3M Espe, St. Paul, USA; 4 Seasons A3 Enamel, A3 Dentin and High Value, Ivoclar Vivadent, Liechtenstein) and one specimen of a silorane-based material (Filtek P90 A3, 3M Espe, St. Paul, USA) were constructed with the aid of a polytetrafluorethane matrix (10 mm of diameter and 2 mm of thickness).
The matrix was placed onto a microscope lamina of 1 mm of thickness, followed by the insertion with resin composite. Then a polyester strip was placed onto the orifice of the matrix and another microscope lamina of the same thickness was positioned onto it. Next the resin composite was light-cured for 40 s with a LED device (Translux Power Blue, Heraeus Kulzer GmbH, Hanau, Germany) with light intensity of 1000 mW/cm2. The device intensity was determined prior to the beginning of the tests with the aid of a radiometer linked to it. The specimens were manually polished with decreasing sequence of polishing discs (Sof-Lex, 3M Espe, St. Paul, USA). A single operator polished all the specimens and each disc was used for 15 s. The specimens were stored into distilled water at 37ºC within dark flasks for 1 h, 24 h or seven days. After each storage time, the specimens were kept for 10 minutes in a cola-based soft drink. The color was evaluated by the CIE-Lab system with the aid of a spectrophotometer (VITA Easyshade 3D Master, Vita Zahnfabrik, Bäd Sackingen, Germany).
The color variation (ΔE) was calculated from the individual values of L*, a* and b*, according to the following formula:
ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2 (1)
In which ΔL*, Δa* and Δb* represents the differences between the readings of the color parameters obtained from the specimens at the different conditions evaluated.
The color variation was considered imperceptible when < 1, clinically acceptable when ≤ 3.3 and unacceptable when higher than 3.3 23,26.
The data were evaluated through one-way ANOVA and Dunnett's T3 test (α = 0.05).
Results
The means and standard deviations of the data obtained from the calculation of ΔE can be observed in table I and in figure 1. It was observed differences among the resins (p < 0.001) and an interaction effect among the resins and the storage time (p < 0.001). The time was not significant (p = 0.246). P90 exhibited a ΔE smaller than 1 at all times studied. Supreme XP CT and 4 Seasons High Value showed a higher ΔE, without surpassing the critical value 3.3. The single material presenting a ΔE greater than 3.3 was Opallis DA3 after the 1 h immersion in the dye.
Discussion
The color stability is an important property which can play a determinant role in the clinical behavior of the resin composite restorations. This aspect is among one of the most used criteria of clinical evaluation in Dentistry: the United States Public Health Service (USPHS). Initially proposed by Ryge 24, this system of evaluation establishes the color and the marginal discoloration as two determinant factors of in vivo longevity of the restorations.
ISO 7491:2000 resolution has been commonly employed in the analysis of the color stability of resin-based materials 27. According to this resolution, resin composite specimens are exposed to a xenon gas lamp with illuminance of 150 klx for a period of 24 h. The color is evaluated before and after the light exposure through the visual scale or colorimeters. However, this artificial aging method not even reproduces the clinical conditions, once the restorations performed within the oral cavity are exposed to limited amounts of light with wavelengths at the ultraviolet area. For this reason, this present study assessed the color after the exposure of different resins to a cola-based soft drink, which can be considered as more clinically relevant. When spectrophotometers or colorimeters are utilized for color analysis, one can calculated the color variation (ΔE) objectively, through the CIE-Lab color system (Commision Internationale de l'Eclairage, L*, a*, b*). Other systems or color spaces such as Munsell's could be employed 6. However, the CIE-Lab system is the most employed because it allows the comparison of the results with those of the clinical visualization 18. The CIE-Lab system defines the color tridimensionally, because the parameter L* corresponds to the luminosity degree, a* corresponds to red or green (+a* = red and –a* = green) and b* yellow or blue (+b*= yellow and –b* = blue) 16,22,23. It has been considered that a color variation lower than 1 is not clinically perceptible 26, it is acceptable when it ranges from 1 to 3.3 and unacceptable when it is higher than 3.3 23.
The color of a resin composite restoration can be affected by chemical differences among the components of the resin phase, such as the monomers and the photo-initiator system 4,10,25. In this present study, the silorane-based resin exhibited a ΔE smaller than 1 at all time periods studied. These data are in agreement with those of another study evaluating the color stability of a silorane-based resin after the artificial aging with exposure to light and xenon at 150 klx for 192 h 12. One possible explanation for these data can be the different chemical composition of the resin matrix compared with the methacrylate-based resins. It has been reported that silorane-based materials have good chemical stability when exposed to aqueous environment 9, in addition to a low solubility and water sorption 19. Conversely to the results of this study, other research assessing the color of a silorane-based resin found a mean ΔE of 18.6 units after 384 h of artificial aging 21.
Together with the silorane-based resin, resins composed of different opacities were evaluated in this present study. The color of a resin composite can be influenced by the features of light transmittance 2. The transmittance means the luminous energy fraction which can surpass a determined material thickness, without being absorbed. It is measured as percentage, relatively to the amount of energy and wavelength of the incident light radiation 5. This optic feature is closely linked to the opacity of the material. Supreme XT CT and 4 Seasons High Value showed the highest ΔE, without surpassing the critical value of 3.3.
Despite the fact that only one material displayed a ΔE higher than 3.3 units at a post-polymerization time of only 1 hour, caution must be taken when one interprets the results of this present study. ΔE values higher than 1 were observed many times, meaning that even 24 h to seven days after the polymerization, it can still have color variation capable of being perceived visually if marked exposures to dyes would occur. Thus, after the construction of resin composite restorations it is necessary to instruct the patients that: 1) it is important to keep adequate oral hygiene habits; 2) the esthetics of the restorations can be longer maintained if some restriction of the consumption of food containing dyes were taken 1.
Conclusion
Given the results of this study, it can be concluded that the time was not significant and the silorane-based material showed the smallest ΔE at the time periods studied.
References
1. Ardu S, Braut V, Gutemberg D, Krejci I, Dietschi D, Feilzer AJ. A long-term laboratory test on staining susceptibility of esthetic composite resin materials. Quintessence Int. 2010 Sep;41(8):695-702. [ Links ]
2. Arikawa H, Fujii K, Kanie T, Inoue K. Light transmittance characteristics of light-cured composite resins. Dent Mater. 1998 Nov;14(6):405-11. [ Links ]
3. Arocha MA, Mayoral JR, Lefever D, Mercade M, Basilio J, Roig M. Color stability of siloranes versus methacrylate-based composites after immersion in staining solutions. Clin Oral Investig. 2012 Sep;20. [ Links ]
4. Asmussen E. Factors affecting the color stability of restorative resins. Acta Odontol Scand. 1983;41(1):11-8. [ Links ]
5. Atkins P, Paula J. Molecular spectroscopy 1: rotational and vibrational spectra. In: Atkins P, Paula J, editors. Atkins' physical chemistry. 8. ed. Oxford: Oxford University Press; 2006. p. 430-80. [ Links ]
6. Barutcigil C, Yildiz M. Intrinsic and extrinsic discoloration of dimethacrylate and silorane based composites. J Dent. 2012 Jul;40(Suppl 1):e57-63. [ Links ]
7. Darvell BW. Materials science for dentistry. 7. ed. Hong Kong: B W Darvell; 2002. [ Links ]
8. Douglas RD. Color stability of new-generation indirect resins for prosthodontic application. J Prosthet Dent. 2000 Feb;83(2):16666-70. [ Links ]
9. Eick JD, Smith RE, Pinzino CS, Kostoryz EL. Stability of silorane dental monomers in aqueous systems. J Dent. 2006 Jul;34(6):405-10. [ Links ]
10. Ferracane JL. Correlation between hardness and degree of conversion during the setting reaction of unfilled dental restorative resins. Dent Mater. 1985 Feb;1(1):11-4. [ Links ]
11. Fontes ST, Fernandez MR, de Moura CM, Meireles SS. Color stability of a nanofill composite: effect of different immersion media. J Appl Oral Sci. 2009 Sep-Oct;17(5):388-91. [ Links ]
12. Furuse AY, Gordon K, Rodrigues FP, Silikas N, Watts DC. Colour-stability and gloss-retention of silorane and dimethacrylate composites with accelerated aging. J Dent. 2008 Nov;36(11):945-52. [ Links ]
13. Furuse AY, Mondelli J, Watts DC. Network structures of Bis-GMA/TEGDMA resins differ in DC, shrinkage-strain, hardness and optical properties as a function of reducing agent. Dent Mater. 2011 May;27(5):497-506. [ Links ]
14. Guggenberger R, Weinmann W. Exploring beyond methacrylates. American Journal of Dentistry. 2000 Nov;13(Spec No):82D-4D. [ Links ]
15. Ilie N, Hickel R. Resin composite restorative materials. Aust Dent J. 2011 Jun;56(Suppl 1):59-6666. [ Links ]
16. Joiner A. Tooth colour: a review of the literature. J Dent. 2004;32(Suppl 1):3-12. [ Links ]
17. Kang A, Son SA, Hur B, Kwon YH, Ro JH, Park JK. The color stability of silorane- and methacrylate-based resin composites. Dent Mater J. 2012;31(5):879-84. [ Links ]
18. O'Brien WJ, Hemmendinger H, Boenke KM, Linger JB, Groh CL. Color distribution of three regions of extracted human teeth. Dent Mater. 1997 May;13(3):179-85. [ Links ]
19. Palin WM, Fleming GJP, Burke FJT, Marquis PM, Randall RC. The influence of short and medium-term water immersion on the hydrolytic stability of novel low-shrink dental composites. Dent Mater. 2005;21:852-63. [ Links ]
20. Peutzfeldt A. Resin composites in dentistry: the monomer systems. Eur J Oral Sci. 1997 Apr;105(2):97-116. [ Links ]
21. Pires-de-Souza FC, Garcia LF, Roselino LM, Naves LZ. Color stability of silorane-based composites submitted to accelerated artificial ageing – an in situ study. J Dent. 2011 Jul;39(Suppl 1):e18-24.
22. Rosenstiel SF, Porter SS, Johnston WM. Colour measurements of all ceramic crown systems. J Oral Rehabil. 1989 Sep;16(5):491-501. [ Links ]
23. Ruyter IE, Nilner K, Moller B. Color stability of dental composite resin materials for crown and bridge veneers. Dent Mater. 1987 Oct;3(5):246-51. [ Links ]
24. Ryge G. Clinical criteria. Int Dent J. 1980 Dec;30(4):347-58. [ Links ]
25. Schneider LF, Pfeifer CS, Consani S, Prahl SA, Ferracane JL. Influence of photoinitiator type on the rate of polymerization, degree of conversion, hardness and yellowing of dental resin composites. Dent Mater. 2008 Mar;24(9):1169-77. [ Links ]
26. Seghi RR, Johnston WM, O'Brien WJ. Performance assessment of colorimetric devices on dental porcelains. J Dent Res. 1989 Dec;68(12):1755-9. [ Links ]
27. EN ISO 7491: Dental materials – determination of colour stability. 2000.
28. Téo TB, Takahashi MK, Gonzaga CC, Lopes MGK. Avaliação, após clareamento, da alteração de cor de dentes bovinos imersos em soluções com elevado potencial de pigmentação. RSBO. 2010;7(4):401-5. [ Links ]
29. Tonetto MR, Santezi Neto C, Felício CM, Domingos PAS, Campos EA, Andrade MF. Effect of staining agents on color change of composites. RSBO. 2012;9(3):26666-71. [ Links ]
30. Tuncdemir AR, Aykent F. Effects of fibers on the color change and stability of resin composites after accelerated aging. Dent Mater J. 2012;31(5):872-8. [ Links ]
31. Xie P, Lu J, Wan H, Hao Y. Effect of toothpaste containing d-limonene on natural extrinsic smoking stain: a 4-week clinical trial. Am J Dent. 2010 Aug;23(4):196-200. [ Links ]
 Corresponding author:
Corresponding author:
Adilson Yoshio Furuse
Universidade Positivo – Pós-graduação em Odontologia
Rua Professor Pedro Viriato Parigot de Souza, n. 5.300 – Campo Comprido
CEP 81280-330 – Curitiba – PR – Brasil
E-mail: ayf@up.com.br
Received for publication: November 12, 2012
Accepted for publication: December 19, 2012













