Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.4 Joinville Out./Dez. 2013
ORIGINAL RESEARCH ARTICLE
Bone apposition and surface treatment in dental implants: histomorphometric pilot evaluation in rabbits
Marcos Nunes Lourenço I; Elisa Mattias Sartori II; Luis Eduardo Marques Padovan III; Geninho Thomé I; Rafael Silveira Faeda IV; Elcio Marcantonio Jr. V; Marcela Claudino I
I Department of Post-Graduation, Latin American Institute of Dental Research and Education – Curitiba – PR – Brazil
II Department of Surgery, Camilo Castelo Branco University – Fernandópolis – SP – Brazil
III Department of Surgery and Implantology, Sagrado Coração University – Bauru – SP – Brazil
IV Department of Periodontology, Dental School of Araraquara University Center – Araraquara – SP – Brazil
V Department of Oral Diagnosis and Surgery, Araraquara Dental School, Sao Paulo State University – Araraquara – SP – Brazil
ABSTRACT
Introduction: The surface of dental implants is an important factor for osseointegration process and different methods of surface treatment have been described. Objective: To investigate the bone apposition in implant surface treated with sandblasting and acid-etching. Material and methods: Ten rabbits were selected and received one implant treated with method I in the left tibia and one implant treated with method II in the right tibia. Then, twenty implants were divided in two groups, according to methods of sandblasting and acid-etching (method I and method II). After 7, 14, 30, 45 and 60 days, tibias were retrieved and submitted to histotechnical procedures. The percentages of bone–implant contact (BIC) and bone area between threads (BABT) were determined throughout histomorphometric analysis and bone apposition was detected in implants of both groups. Results: In BABT measurements, an increase was observed after 45 and 60 days in the method II, compared to method I and no differences were found after 7, 14 and 30 days. In BIC measurements, an increase was detected with method II at 45 days when compared to method I. No differences between groups in BIC values were observed after 7, 14, 30 and 60 days. Conclusion: Our data demonstrated that implants treated with the method II presented increase in the contact between bone and implant after 45 days compared to method I. Moreover, with concern to bone area between threads, it was observed an increased in the method II after 45 and 60 days. However, both groups can be successfully used as a therapeutic strategy to rehabilitation of edentulous patients. Then, further experiments are needed to evaluate, in depth, the putative differential role of each surface treatment.
Keywords: osseointegration; dental implant; osteogenesis; titanium.
Introduction
Dental implants have been considered a safe and predictable treatment for replacing missing teeth to restore function in partially or completely edentulous patients. However, the success of this treatment is associated to osseointegration, which is defined as a direct connection between living bone and the surface of implant without interposed soft tissue. It is an anchorage mechanism whereby synthetic components can be incorporated into living bone, persisting under all normal conditions of loading. Therefore, osseointegration process is strictly related to bone metabolism 1,5,8,24.
Different types of cells participate in the bone metabolism, such as osteoblasts, osteoclasts and osteocytes. Osteoblasts are specialized cells that reside in the bone surface and are responsible for the synthesis of the bone extracellular matrix and influence bone mineralization while osteoclasts are responsible for bone resorption 32. In this way, bone is resorbed and replaced in a physiological process characterized as bone remodeling 39. This process also occurs when an implant is placed 17,18,25 and the clinical success of oral implants is related with their osseointegration 24.
This peri-implant bone formation can be divided in contact and distant osteogenesis. Contact osteogenesis is characterized by deposition of a newly formed bone in direct contact with the implant surface. Regarding to distant osteogenesis, bone tissue is deposited on the surface of old bone in the peri-implant site and the bone surface provided a population of osteogenic cells. The new bone is not deposited on the implant; however, the implant does become surrounded by bone 23,25.
Many factors could affect the osseous healing of implants such as surface topography of biomaterial, the status of the bone/implant site, implant loading conditions, surgical technique and implant design 1,3,15,21. Considering that surface topography, implant design and surface seem to influence the bone apposition, numerous studies demonstrated that the surface roughness of titanium implants affects the rate of osseointegration 16,22,37,43.
Several methods have been used to create a rough surface and improve the osseointegration of titanium implants such as titanium plasma-spraying, blasting with ceramic particles, acid-etching and anodization 24. Many studies have been developed in order to evaluate the bone deposition in dental implant treated with different methods of surface treatment 2,11,12. Although some studies demonstrated that different methods of implant surface treatment affect the host-to-implant response, it would be reasonable to evaluate the differences between different methods of sandblasting and acid-etching. Therefore, the aim of this pilot study was to investigate the putative differences in the pattern of bone apposition in implants treated with different techniques of sandblasting and acid-etching using the rabbit tibia model after 7, 14, 30, 45 and 60 days after implant placement.
Material and methods
Implants and experimental animals
Ten New Zealand white mature male rabbits with a mean weight of 4 kg were used in this study. The animals were kept in individual cages, fed with a standard laboratory diet and given tap water ad libitum. Initially, ten animals were randomly divided in two groups, according to the surface treatment. In the sequence, rabbits were subdivided into 5 subgroups, according to experimental periods (7, 14, 30, 45 and 60 days after surgical procedures). Each animal received two implants, one implant in the right tibia and one implant in the left tibia. Therefore, each experimental group was composed by 2 samples, characterizing a pilot study. These procedures were performed under sterile conditions and the study protocol was approved by the Sagrado Coração University Ethics Committee, USC (016/09).
All implants were manufactured from commercially pure (grade IV) titanium. According to surface treatment methods, the implants were divided into two groups. In group I, implants (10 mm long and 3.3 mm in diameter) were placed in left tibia of each animal. The method I of surface treatment was produced by a large grit sandblasting process with corundum particles that leads to a macro roughness on the titanium surface. This is followed by a strong acid-etching bath with a mixture of HCl/H2SO4, producing the fine 2-4 m micropits superimposed on the rough-blasted surface, as described by the manufacturer (Straumann AG, Basel, Switzerland). In the method II, implants with 10 mm long and 3.5 mm in diameter were placed in left tibia of each rabbit. The surface of this group was produced by aluminum oxide sandblasting. The next step was characterized by immersion in acid solution for long periods in high temperature, resulting in 2.5-5 m micropits, as described by the manufacturer (Neodent, Curitiba, Brazil).
Surgery and histological procedures
Prior to surgery, the shaved skin in the tibial metaphysis area was cleaned with iodine solution at the surgical and surrounding area. The animals were anaesthetized through intramuscular injection of a combination of ketamine (Ketamina Agener®; Agener União Ltda., São Paulo, SP, Brazil) (0.35 mg/kg of body weight) and xylazine (Rompum® Bayer S.A. São Paulo, SP, Brazil) (0.5 mg/kg of body weight). Incisions of approximately 3 cm in length were performed in the left and right tibiae. After dissection, the bone surface of the tibial metaphysis was exposed and one implant was placed in each tibia. Implants were placed using a progressive sequence of drills under saline cooling, according to the manufacturer's instructions. The soft tissues were sutured in separate layers and the animals received postoperatively a single intramuscular dose of antibiotic (Pentabiótico Pequeno Porte – Fort Dodge®, Campinas, SP, Brazil) (0.1 ml/kg of body weight).
After 7, 14, 30, 45 and 60 days, animals were sacrificed by intramuscular injection of high dose of the anesthetic solution and the tibiae containing the implants were removed in terms of histological techniques. Tissue blocks containing the implant were fixed in 10% buffered formalin solution for 24h, washed in running water for 24 hours and dehydrated in a series of alcohol solutions ranging from 70-100% ethanol. Following dehydration, the samples were embedded in a methacrylate-based resin (Technovit 9100, Heraeus Kulzer GmbH, Wehrheim, Germany) according to the manufacturer's instructions. In the sequence, the blocks were sectioned using a diamond saw (Exakts, Apparatebau GmbH, Norderstedt, Germany) and sections (~300 m thickness) were glued to acrylic plates with an acrylate-based cement, and a 24 hours setting time was allowed prior to grinding and polishing. The sections were then reduced to a final thickness of ~30 m by means of a series of SiC abrasive papers (400, 600, 800, 1200 and 2400 grit) (Buehler Ltd., Lake Bluff, IL, USA) in a grinding/polishing machine (Metaserv 3000, Buehler Ltd., Lake Bluff, USA) under water irrigation. The sections were stained with Stevenel's blue and acid fuchsin. All histological procedures were performed in Exakt System Laboratory, Araraquara School of Dentistry – UNESP.
Histological analysis
All histological sections were identified with a random numerical sequence in order to codify experimental periods and groups. Histomorphometric evaluation was performed using an optical microscope (Axion Imager A1M, Carl Zeiss, Germany) attached to a digital camera (Axiocam ICc3, Carl Zeiss, Germany). The acquired digital images were analyzed by a single and calibrated examiner (MC) blind to experimental groups and periods. Osseointegration process was evaluated throughout measurements of bone-to-implant contact (BIC) and mineralized bone area between threads (BABT) using the software Image Tool 3.0 (San Antonio Dental School, University of Texas Health Science, TX, USA).
The regions of bone-to-implant contact (BIC) along the implant perimeter were subtracted from the total implant perimeter and the calculations were performed to determine the BIC. In bone area between threads (BABT), we firstly obtained the total area of threads and the area occupied by space or no-bone, and after we determine the percentage of total area of threads occupied by bone tissue. These assessments were performed bilaterally in the first three threads of each implant by a single calibrated examiner.
Statistical analysis
Data are presented as mean ± SD, and the statistical differences between experimental groups were analyzed by ANOVA, followed by Tukey test. The intra-examiner casual error was calculated with paired "t" test and the systematic error was calculated according to Dahlberg formula. Both analyses were performed by GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Values of p<0.05 were considered statistically significant.
Results
The surgical procedures and follow-up demonstrated no complications regarding procedural conditions, post-operative infection or other clinical concerns. No implants were excluded from the study due to clinical instability and no clinical signs of inflammation were detected in any of the specimens. Our histological findings revealed a relevant pattern of bone deposition, demonstrating the occurrence of osseointegration in both groups (figures 1 and 2). However, significant differences between method I and II were observed with regard to bone-to-implant contact (BIC) and mineralized bone area between threads (BABT).
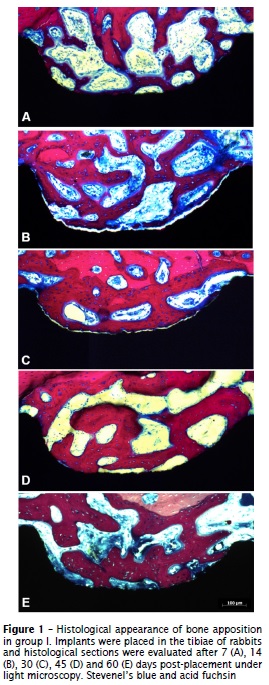
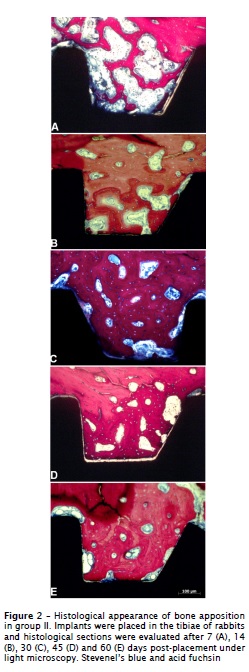
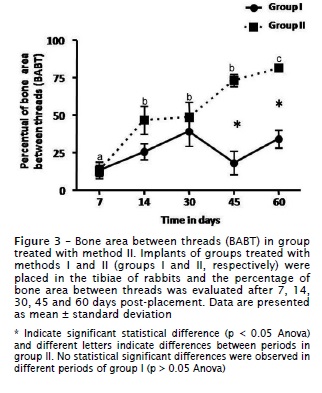
The morphometric analysis did not demonstrated statistical differences between methods I and II at 7, 14 and 30 days of healing (p > 0.05). However, it was demonstrated a significant increase (p < 0.05) in the bone area between threads (BABT) after 45 and 60 days in the method II. After 45 days of healing, the mean BABT was 73.1% ± 15 for the method II and 17.4% ± 26.2 for the method I. At 60 days of healing, BABT measurements of method II averaged 81.9% ± 5.4 and 33.2% ± 20 for method I (figure 3). Evaluating the values of BABT in different periods of method II, we observed a progressive increase (p < 0.05) in their measurements along healing process (figure 3). Conversely, we did not observe significant differences in BABT measurements (p > 0.05) in all time points of group treated with method I.
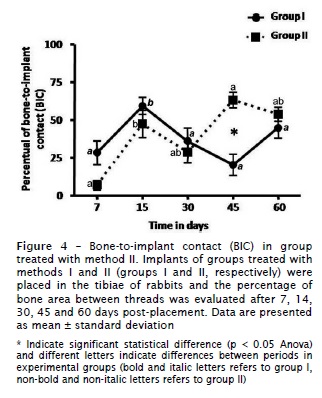
Significant differences in percentage of bone-to-implant contact (BIC) between methods I and II were observed at 45 days of healing, demonstrating an increase (p < 0.05) in BIC values in the method II (figure 4). After 45 days, BIC values were 60.2% ± 20.6 for the method II and 21.2% ± 23.2 for the method I. There was no statistically significant difference between groups in regards to bone-to-implant contact after 7, 14, 30 and 60 days (p > 0.05). Our data also demonstrated significant difference in BIC measurements in different time-points in groups treated with methods I and II, as illustrated in figure 4.
Intra-examiner casual and systematic error did not show statistical significant difference between measurements, demonstrating reproducibility and reliability of the method. The casual error was considered low, being 2.6% for BABT measurements and 1.8% for BIC values. For systematic error, we did not found statistical difference (p > 0.05) among repeated measurements regarding to BABT and BIC analyses.
Discussion
Surface roughness is one of the key factors for osseointegration of titanium dental implants. It is well established that surface modifications can enhance bone integration of titanium implants in comparison with polished titanium surface 7,19,26,35,41. In fact, increased surface roughness of dental implants resulted in greater bone apposition 6 and reduced healing time 10. Several methods are used to perform a surface treatment in dental implants 24; however the pattern of bone deposition in different techniques of sandblasting and acid-etching remains poorly known. In this way, the purpose of this study was to evaluate these possible differences using the rabbit tibia model, which has been largely used in experimental studies 20,29,34.
Our results showed evidences of success in osseointegration process, considering that both groups treated with different methods of sandblasting and acid-etching presented relevant amounts of bone in the surface of implants. Indeed, a previous study compared two types of dental implant surface (SLActive® and NanoTite™) with concern to peri-implant bone healing after 4 and 8 weeks. The results demonstrated that both types of implants presented satisfactory and similar bone response in the mandible of dogs. However, the authors also suggest that the quality and quantity of bone site could not allow demonstrating significant differences in the healing response 2.
Thus, other authors evaluated the bone regeneration in dehiscence-type defects at titanium implants with chemically modified sandblasted/acid-etched (modSLA) or dual acid-etched surfaces with a calcium phosphate nanometer particle modification (DCD/CaP). It was observed higher mean of BIC in the modSLA implants, suggesting that these implants may have a higher potential to support osseointegration when compared to DCD/CaP implants 33.
Moreover, all titanium implants placed in tibia of rabbits in our experiments did not present any complication such as clinical instability or clinical signs of exacerbated inflammatory responses. According with our data, several studies have described titanium as an excellent biomaterial and its biocompatibility is related to the formation of a thin surface layer of oxide, being associated with osseointegration mechanisms. The initial reaction of the oxide layer with biological environment is the adsorption of ions and macromolecules, resulting in the formation of a protein-dominated film. These interactions between mineral in the interface and the oxide layer seem to contribute to the anchoring of the implant, resulting in bone apposition 4,14. Nevertheless, this biological process can be influenced by several characteristics of dental implants such as surface topography, implant design and finish; all of which are really relevant to the osseointegration 3,15,21,24,37.
Our results also demonstrated variations in the amount of bone deposited among groups characterized by an increase in the area between threads occupied by bone tissue and bone-implant contact in group treated with method II. An increase was detected in the area between threads (BABT) occupied by bone tissue after 45 and 60 days of implant placement, accompanied by an increase in bone-implant contact (BIC) after 45 days. Considering that other variables such as surgery technique and implant length were controlled in our experimental design, these findings may be attributed to the specific characteristics of sandblasting and acid-etching in each group. Accordingly, previous studies have reported that bone deposition at the tissue-implant interface is influenced by several factors such as design, chemistry, topography and wettability of dental implant 9,15,23,37.
The design of an implant refers to the three dimensional structure characterized by many terms such as shape, presence or absence of threads, threads design, surface topography and chemical composition. These characteristics affect the biomechanical load distribution on the implant, having an effect in bone deposition and resorption 9,37. Therefore, some studies demonstrated that different implant designs presented different stress distribution in the bone, affecting bone metabolism 36,42. It is possible that slight differences in the implant diameter as well as in the threads design may be influenced our results. However, the influence of macrostructure seems to be minimized in our results, since the implants were not submitted to load in the osseointegration period. Insertion torque values are also important, considering the putative influence in the BIC and BABT evaluations mainly in the first period. In fact, we established a minimum insertion torque values of 10 N.cm in order to reduce this influence.
Considering that both methods are really similar, these variations in the bone apposition seem to be a result of minor differences in manufacturing process of sandblasting and acid-etching between groups such as composition, time and concentration of acids used in the surface treatment. According to manufacturer, titanium implants treated with method I were submitted to a immersion for several minutes in a mixture of concentrated HCl and H2SO4 heated above 100°C, producing the fine 2-4 μm micropits superimposed on the rough-blasted surface. In addition, the method I of surface treatment also comprised an immersion for long periods in acidic solution at high temperature, producing 2.5-5 μm micropits.
Therefore, it has been described that implant surface interacts with the host, having a direct role in osteogenesis at the bone-implant interface, influencing a series of coordinated events including protein adsorption, cell proliferation, and bone tissue deposition 23,27,40. In fact, acid-etched surfaces enhance the osteoconductive process through the attachment of fibrin and osteogenic cells, resulting in bone formation directly on the surface of the implant 28. Then, specific surface properties of sandblasted-acid-etched implants may modulate the biological behavior of osteoblasts during bone tissue healing 13,30. In this way, it is possible to suggest that minor differences in surface microstructure may induce a delay in the adhesion, proliferation and differentiation of osteoblastic cells on the titanium surface and may explain the differences between groups.
Although some differences in BABT and BIC, our data demonstrated similarity in the kinetics of osseointegration in both groups, mainly in BIC values. We initially observed an increase in BIC values at 14 days, followed by a transitory reduction with a posterior recovery in the last period. In this initial periods (7 and 14 days), it was observed a trend towards the increase in BIC values in group treated with method I, suggesting a possible improvement in early phases of tissue healing. In the sequence, the phase characterized by reduction in BIC was longer (30 and 45 days) in method I when compared to method II (30 days); however we did not found any differences between groups at the end of experimental times (60 days) between groups. Then, it is possible to suggest that the method I presented a transitory delay in osseointegration process, possibly due by changes in biological mechanisms such as protein adsorption, cell proliferation and bone tissue deposition 23,27,39. Our hypothesis could be reinforced by a reduction in bone apposition in method I at 45 and 60 days in the bone area between threads (BABT), parameter that have been considered as valuable for evaluating osseointegration 31,38. The difference of the bone area between threads seems to be related to differences in the macrostructure of the implant. In fact, it has been previously demonstrated that these characteristics may affect the biomechanical distribution, influencing the pattern of bone deposition 9,37.
Conclusion
Our data demonstrated that implants treated with the method II presented increase in the contact between bone and implant after 45 days compared to method I. Moreover, with concern to bone area between threads, it was observed an increased in the method II after 45 and 60 days. However, although slight differences in bone apposition, dental implants from both groups can be considered as a very interesting and predicable strategy to oral rehabilitation of missing teeth. Possibly, slight variations in surface treatment may affect the osseointegration process. Whereas it is well established that surface roughness and design of implants plays an important role for cellular reactions and tissue healing, the precise effect of these characteristics and the kinetics of biological mechanisms involved in osseointegration remains poorly understood. Moreover, it would be interesting to evaluate the pattern of bone apposition in both methods after longer periods of implant placement. Then, further studies must be carried out in order to improve knowledge on the interaction between osseointegration and implant surface treatment, which may serve as a basis for development of more effective strategies for improve the performance of titanium implants.
References
1. Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;110(6):387-416. [ Links ]
2. Al-Hamdan K, Al-Moaber SH, Junker R, Jansen JA. Effect of implant surface properties on peri-implant bone healing: a histological and histomorphometric study in dogs. Clin Oral Implants Res. 2011;22(4):399-405.
3. Bahat O, Sullivan RM. Parameters for successful implant integration revisited part I: immediate loading considered in light of the original prerequisites for osseointegration. Clin Implant Dent Relat Res. 2010;12 Suppl 1:e2-12.
4. Baier RE, Meyer AE, Natiella JR, Natiella RR, Carter JM. Surface properties determine bioadhesive outcomes: methods and results. J Biomed Mater Res. 1984;18(4):327-55.
5. Brånemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38(2):175-81.
6 . Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83(7):529-33.
7. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25(7):889-902.
8. Carlsson L, Röstlund T, Albrektsson B, Albrektsson T, Brånemark PI. Osseointegration of titanium implants. Acta Orthop Scand. 1986;57(4):285-9.
9. Cehreli M, Sahin S, Akça K. Role of mechanical environment and implant design on bone tissue differentiation: current knowledge and future contexts. J Dent. 2004;32(2):123-32.
10. Cochran DL, Buser D, ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP et al. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002;13(2):144-53.
11. Coelho PG, Bonfante EA, Pessoa RS, Marin C, Granato R, Giro G et al. Characterization of five different implant surfaces and their effect on osseointegration: a study in dogs. J Periodontol. 2011;82(5):742-50.
12. Coelho PG, Granjeiro JM, Romanos GE, Suzuki M, Silva NR, Cardaropoli G et al. Basic research methods and current trends of dental implant surfaces. J Biomed Mater Res B Appl Biomater. 2009;88(2):579-96.
13. Conserva E, Lanuti A, Menini M. Cell behavior related to implant surfaces with different microstructure and chemical composition: an in vitro analysis. Int J Oral Maxillofac Implants. 2010;25(6):1099-107.
14. Damen JJ, Ten Cate JM, Ellingsen JE. Induction of calcium phosphate precipitation by titanium dioxide. J Dent Res. 1991;70(10):1346-9.
15. Depprich R, Zipprich H, Ommerborn M, Naujoks C, Wiesmann HP, Kiattavorncharoen S et al. Osseointegration of zirconia implants compared with titanium: an in vivo study. Head Face Med. 2008;11(1):30-4.
16. Elias CN, Meirelles L. Improving osseointegration of dental implants. Expert Rev Med Devices. 2010;7(2):241-56.
17. Fleischmannova J, Matalova E, Sharpe PT, Misek I, Radlanski RJ. Formation of the tooth-bone interface. J Dent Res. 2010;89(2):108-15.
18. Franchi M, Fini M, Martini D, Orsini E, Leonardi L, Ruggeri A et al. Biological fixation of endosseous implants. Micron. 2005;36(7):66665-71.
19. Gotfredsen K, Berglundh T, Lindhe J. Anchorage of titanium implants with different surface characteristics: an experimental study in rabbits. Clin Implant Dent Relat Res. 2000;2(3):120-8.
20. He FM, Yang GL, Zhao SF, Cheng ZP. Mechanical and histomorphometric evaluations of rough titanium implants treated with hydrofluoric acid/nitric acid solution in rabbit tibia. Int J Oral Maxillofac Implants. 2011;26(1):115-22.
21. Javed F, Almas K, Crespi R, Romanos GE. Implant surface morphology and primary stability: is there a connection? Implant Dent. 2011;20(1):40-6.
22. Junker R, Dimakis A, Thoneick M, Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(4):185-206.
23. Lavenus S, Louarn G, Layrolle P. Nanotechnology and dental implants. Int J Biomater. 2010:915327.
24. Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844-54.
25. Marco F, Milena F, Gianluca G, Vittoria O. Peri-implant osteogenesis in health and osteoporosis. Micron. 2005;36(7):630-44.
26. Marinho VC, Celletti R, Bracchetti G, Petrone G, Minkin C, Piattelli A. Sandblasted and acid-etched dental implants: a histologic study in rats. Int J Oral Maxillofac Implants. 2003;18(1):75-81.
27. Palmquist A, Omar OM, Esposito M, Lausmaa J, Thomsen P. Titanium oral implants: surface characteristics, interface biology and clinical outcome. J R Soc Interface. 2010;7(5):515-27.
28. Park JY, Davies JE. Red blood cell and platelet interactions with titanium implant surfaces. Clin Oral Implants Res. 2000;11(6):530-9.
29. Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13(1):1-10.
30. Ramaglia L, Postiglione L, Di Spigna G, Capece G, Salzano S, Rossi G. Sandblasted-acid-etched titanium surface influences in vitro the biological behavior of SaOS-2 human osteoblast-like cells. Dent Mater J. 2011;30(2):183-92.
31. Roriz VM, Rosa AL, Peitl O, Zanotto ED, Panzeri H, de Oliveira PT. Efficacy of a bioactive glass-ceramic (Biosilicate) in the maintenance of alveolar ridges and in osseointegration of titanium implants. Clin Oral Implants Res. 2010;21(2):148-55.
32. Scheller EL, Krebsbach PH. The use of soluble signals to harness the power of the bone microenvironment for implant therapeutics. Int J Oral Maxillofac Implants. 2011;26(2):70-9.
33. Schwarz F, Sager M, Kadelka I, Ferrari D, Becker J. Influence of titanium implant surface characteristics on bone regeneration in dehiscence-type defects: an experimental study in dogs. J Clin Periodontol. 2010;37(5):46666-73.
34. Seong WJ, Grami S, Jeong SC, Conrad HJ, Hodges JS. Comparison of push-in versus pull-out tests on bone-implant interfaces of rabbit tibia dental implant healing model. Clin Implant Dent Relat Res. 2013;15(3):460-9.
35. Shalabi MM, Gortemaker A, Van't Hof MA, Jansen JA, Creugers NH. Implant surface roughness and bone healing: a systematic review. J Dent Res. 2006;85(6):496-500.
36. Siegele D, Soltesz U. Numerical investigations of the influence of implant shape on stress distribution in the jaw bone. Int J Oral Maxillofac Implants. 1989;4(4):333-40.
37. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000;15(5):675-90.
38. Tavares MG, de Oliveira PT, Nanci A, Hawthorne AC, Rosa AL, Xavier SP. Treatment of a commercial, machined surface titanium implant with H2SO4/H2O2 enhances contact osteogenesis. Clin Oral Implants Res. 2007;18(4):452-8.
39. Vandrovcová M, Bačáková L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol Res. 2011;60(3):403-17.
40. Vasconcellos LM, Leite DO, Oliveira FN, Carvalho YR, Cairo CA. Evaluation of bone ingrowth into porous titanium implant: histomorphometric analysis in rabbits. Braz Oral Res. 2010;24(4):399-405.
41. Wennerberg A, Albrektsson T, Andersson B, Krol JJ. A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin Oral Implants Res. 1995;6(1):24-30.
42. Wennerberg A, Albrektsson T, Andersson B. Design and surface characteristics of 13 commercially available oral implant systems. Int J Oral Maxillofac Implants. 1993;8(6):622-33.
43. Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(4):172-84.
 Corresponding author:
Corresponding author:
Marcela Claudino
Instituto Latino Americano de Pesquisa e Ensino Odontológico – ILAPEO
Rua Jacarezinho, n. 656
CEP 80710-150 – Curitiba – PR – Brasil
E-mail: marcelaclaudino@hotmail.com
Received for publication: July 22, 2013
Accepted for publication: August 7, 2013













