Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.4 Joinville Out./Dez. 2013
ORIGINAL RESEARCH ARTICLE
Implications of the use of bisphosphonates in dental treatment – experience of the service of oral and maxillofacial surgery, Erasto Gaertner Hospital, Curitiba, Brazil
Regiane Benez Bixofis I; Laurindo Moacir Sassi I; Cleverson Patussi I; William Phillip Pereira da Silva I; Roberta Targa Stramandinoli Zanicotti I; Juliana Lucena Schussel I
I Department of Oral and Maxillofacial Surgery, Erasto Gaertner Hospital – Curitiba – PR – Brazil
ABSTRACT
Introduction: Bisphosphonates (BP) are effective drugs in the prevention and treatment of various bone pathologies, acting in the regulation of osteoclast function through different mechanisms. Despite the success in the treatment of various diseases, these drugs have the ability to induce an avascular necrosis of bone tissue, especially in the maxilla and mandible. Objective: Due the significant increase number of cases of patients with oral complications associated with BP therapy in the Department of Oral and Maxillofacial Surgery of the Erasto Gaertner Hospital, the study aimed to report our experience in the care of these patients. Material and methods: Patients submitted to BP therapy were enrolled prospectively between the years of 2011 and 2012. Clinical examination was performed in all patients to evaluate dental health as well oral mucosa. All patients are under follow-up in our service. Results: 26 patients who used BP were attended in the ambulatory. Twenty-three patients used BP for oncological indication and three for osteoporosis. Most of patients were women (6666%) with average age of 56 years old. The most frequent medication used was Pamidronate (54%), followed by Zoledronic acid (30%) and Alendronate (15%). Ten patients showed bone exposition, most of then in the mandible (80%), with an average time of one year of exposure. Conclusion: Dentists should advise their patients about the use of BP and the implications for oral health and treatments. These patients must have periodic consultations for evaluation and early detection of osteonecrosis associated to BP for adequate treatment.
Keywords: bisphosphonates; osteonecrosis; oral complications.
Introduction
Bisphosphonates (BP) are effective drugs in the prevention and treatment of various bone pathologies, such as Paget's disease, hypercalcemia, malignancy, osteolytic lesions in multiple myeloma, pathologic fractures, spinal cord compression, steroid-induced or postmenopausal osteoporosis and bone metastasis associated with solid tumors such as breast, prostate or lung 2,15,16,30. BP act in the regulation of osteoclast function by decreasing bone resorption by different mechanisms, inhibiting the development of their precursor cells, increasing apoptosis rate, stimulating inhibitor factors and reducing its activity 28.
Despite the success in the treatment of various diseases, this drug class has the ability to induce an avascular necrosis of bone tissue, especially in the maxilla and mandible, as initially presented by Marx 13 and Ruggiero et al. 22. The so-called osteonecrosis induced by BP (ONIB) 4 or osteonecrosis associated with BP (OAB) 16 and its occurrence have been studied in patients undergoing oral surgery such as dental extractions, implants installation, grafts, osteogenic distraction, and others 2,4,25. According to the American Association of Oral and Maxillofacial Surgeons (AAOMS), it is difficult to establish the impact of OAB on patients treated with intravenous BP, but it is estimated that the annual incidence varies between 0.8% to 12% 1,17.
Our service noted, in recent years, a significant increase in demand for guidance on complications related to the use of BP and dental treatment. The objective of this study is to report our experience and the protocol used in the care of these patients.
Material and methods
Patients using BP were enrolled prospectively between the years of 2011 and 2012. Most patients had been referred to our service seeking for dental treatment or orientation about dental procedures and systemic conditions. Clinical examination was performed in all patients to evaluate dental health as well oral mucosa. Patients received orientation concerning the use of BP and the implications on oral health and treatments.
Patient's information from medical record was collected including sex, age, primary tumor, occurrence of metastasis, medication, drug administration, time of intake, oral health conditions, use of prosthesis, dental procedure performed and presence of bone exposition.
Results
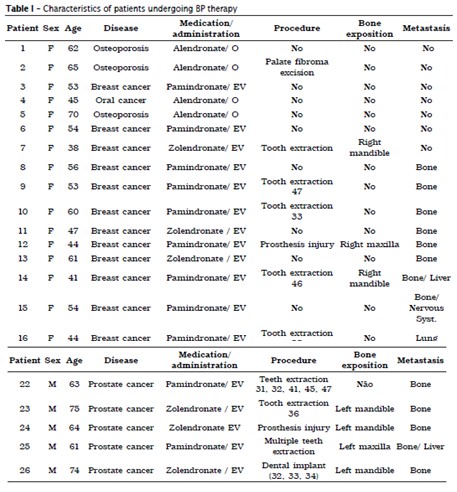
Between the years of 2011 and 2012, 26 patients who used BP were treated in the Service of Oral and Maxillofacial Surgery of the Erasto Gaertner Hospital (table I). The following reasons accounted for their appointments: orientation about dental treatment, physician indication and dental health orientation. Twenty-three patients used BP for oncological indication and three for osteoporosis. Most of patients were women (6666%) with age varying from 38 to 75 years old (average 56 years old). Fourteen patients (54%) had breast cancer as primary tumor, 23% prostate cancer, 7.5% multiple myeloma, 4% oral cancer and 11.5% had osteoporosis. Fifty-seven percent of patients had metastasis history, 27% with bone metastasis, 23% bone and a second site metastasis and 7% with lung metastasis.
The most frequent medication used was Pamidronate (54%), followed by Zoledronic acid (30%) and Alendronate (15%). Eighty-five percent received BP intravenous and 15% orally. The average time of intake was 21.4 months.
Dental condition was evaluated: 15% of patients were edentulous, 11.5% had all teeth and 73.5% had one or more teeth missing. Removable prosthesis use was noticed in 27% of patients and complete denture in 19%. Only one patient had dental implant. 7.6% had poorly adapted prosthesis.
Some patients had performed dental procedures before prior orientation. Among the procedures executed, 38.5% were tooth extraction, 7.6% were dental implants and 4% were lesion removal.
Ten patients showed bone exposition, most of then in the mandible (80%), with an average time of one year of exposure. Five patients had exposure after performing tooth extractions; two after installation and loss of dental implants and 2 had injuries caused due maladapted denture. Only 1 patient developed spontaneous OAB. Four small and medium lesions were observed, ranging between 0.5 and 1.5 cm, asymptomatic, and their treatment included antiseptic mouthwash prescription, bone sequestrum removal when present, and use of analgesic and antibiotic medication. All patients were oriented about the effects of BP medication effects and are monitored regularly.
Discussion
The bone tissue is maintained at a constant homeostatic process through the action of osteoblasts and osteoclasts 28. The constant remodeling occurs in the healthy adult bone in response to physiological stimulations initiated by bone aging, microdamage and stress. The balanced interaction between the different cell types during bone remodeling ensures replacement of defective bone with an equivalent volume of healthy bone. Thus bone mineral density (BMD) and bone strength are preserved 27.
Subramanian et al. 27 believe that OAB occurs after cessation of bone remodeling process, on multiple levels, resulting in an inefficient remodeling that allows the persistence of the bone defect. This situation can be mediated by three main factors: (1) patient condition and the underlying disease, such as osteoporosis, malignant bone disease or Paget's disease; (2) variation of collective impact two precedent factors on bone turnover in the injured site; (3) BP effects 27.
After intravenous or oral administration, a small fraction of BP binds to hydroxyapatite crystals active in remodeling of bone matrix, and the remainder of BP stock is rapidly removed from circulation by the kidneys. The BP fraction bound to the matrix has a half-life of about 11 years and is toxic to the function and survival of osteoclasts. Therefore, the treatment of patients with malignant bone disease or osteoporosis with BP reverses the decompensated resorption and delays bone loss. Patients with osteoporosis demonstrate an improvement in BMD and reduction in fracture incidence, while those with malignant bone disease demonstrate a delay in the occurrence of osteolytic lesions and pathologic fractures 27.
BPs are synthetic analogues of inorganic pyrophosphate, and bind strongly to hydroxyapatite (HAP), depositing on local abundance of the mineral 2,4. According to its structure, can be divided into two classes: non-nitrogenous bisphosphonates (1st generation) and nitrogen (2nd generation) 2,4,15,26. The non-nitrogenous BP acts competing with adenosine triphosphate (ATP) in osteoclasts and activating their process of apoptosis. Because they are rapidly metabolized, its action potential is reduced 15,28.
The nitrogenous BP also induce the process of apoptosis, besides inhibiting the action of farnesyl diphosphate synthase, an enzyme which acts on isoprenoid lipid synthesis interrupting protein binding necessary for osteoclastic function 4,28. Because there is nitrogen in its molecular structure, such BP is not metabolized and accumulates in bone tissue, acting for long periods and therefore having increased potency compared to non-nitrogenous compounds 10.
BP can be used orally, usually recommended either to treat osteoporosis or for oral chemotherapy such as alendronate, risedronate, etidronate, tiludronate, and clodronate. They can also be administered either intravenously or associated with chemotherapy or hormonal therapy, such as zoledronate and pamidronate 2,4,14.
As mentioned above, bisphosphonates are stable analogs of inorganic pyrophosphate. A carbon atom replacing the oxygen atom that connects the two phosphates confers the stability and renders the molecule resistant to biological degradation. All BPs of clinical interest have two phosphate groups that share a common carbon atom (P-C-P) 6.
The two phosphate groups have a dual function; both are required for binding to bone mineral and cell-mediated anti-resorptive activity. Modifications to one or both of the phosphate groups can drastically reduce the affinity for bone mineral BP 5 as well as reduce biochemical power 10.
The R1 and R2 side chains attached to a carbon atom are responsible for the wide range of activity observed among BP 24. The R1 substituents such as hydroxyl or amino improve the mineral chemisorption 29, whereas the substituents R2 results in differences in the anti-resorption power 5,24. Thus, the zoledronate is one of the most potent BPs in anti-resorptive in various animal models due to the nitrogen atom in the heterocyclic ring, whereas alendronate and pamidronate are slightly less potent because a basic primary nitrogen atom in an alkyl chain. The increased anti-resorptive potency observed with the different R2 groups is related to biochemical activity, e.g., inhibition of the farnesyl pyrophosphate synthase (FPPS) enzyme, and is thought to be linked to the ability to bind to hydroxyapatite (HAP) 18.
Nancollas et al. 18 showed significant differences in the binding affinity of various kinetic BP-hydroxyapatite, and set the sort order of clodronate <etidronate <risedronate <ibandronate <alendronate <pamidronate <zoledronate. This same rank order was found to carbonated apatite, which resembles more closely natural bone mineral 8.
The key features of the structure–activity relationships that have been established for the interactions between BPs and HAP show that an OH or NH2 rather than an H group at the R1 site enhances HAP binding, and a nitrogen moiety, and its position in the alkyl group or heterocyclic ring in the R2 side chain, can lead to significant increases in HAP binding 6,18.
The mechanisms of BP responsible for osteonecrosis induction are still not fully understood and may be associated with one or more factors. Its action on bone turnover affects the relationship osteoblast/osteoclast, resulting in compromise of the quality and quantity of newly formed bone tissue, consequently, affects the adaptation and remodeling, causing damage to its microstructure, changing its mechanical properties and making it more vulnerable to bacteria activity in oral cavity 9. As it presents reduced physiological remodeling, the bone becomes brittle and ineffective in the repair of natural microfractures that occur through daily activities 16. Also, the hypothesis that BP antiangiogenic properties compromises blood flow and oxygenation of bone tissue making bone perfusion difficult, and facilitating necrosis 9,17.
Histologically necrotic bone with areas of chronic inflammation represented by mixed cellular infiltrate can be observed 22,23. Bacterial debris may be present 22. Hansen et al. 7 conducted a histomorphological analysis of OAB compared with osteoradionecrosis. In OAB, multiple areas of necrotic bone partially confluent mingled with residual nests of vital bone were present, while in osteoradionecrosis, completely homogeneous regions of necrotic bone were observed. OAB can also highlight, in this type of analysis, a mixed inflammatory infiltrate as well colonies of Actinomyces and Streptococcus in touch with vital bone 2,7.
Some studies showed that, in patients submitted to weekly BP therapy with regular doses for long periods, of three or more years, had an increased risk to OAB development and it is directly proportional to therapy duration 3,4,17,26. OAB is the first late complication of BP therapy described scientifically 13. The highest incidence is associated with chemotherapy in cancer patients who are immunosuppressed, ranging from 1 to 10% of cases, and it is lower in patients who use BP to treat osteoporosis 4,16. Other factors may be implicated in OAB development, as smoking, diabetes, tumor staging, general health condition of the patient, medication as chemotherapy and steroids, oral health status and presence of acute or chronic infection 11,12,16.
OAB consists of an interaction between bone metabolism, local trauma, increased need for bone repair, hypovascularization, and infection 16. Oral OAB is defined as non-vascularized or necrotic bone fragment exposure, in the oral cavity, often combined with inflammation of adjacent tissue and pain 14 for at least 8 weeks, in patient that uses or used BP and who were not head and neck irradiated 16,17.
The maxilla and mandible suffer constant overload due to masticatory forces and microfractures and physiologic microdamage naturally occur, which lose their ability to repair and favor the onset of osteonecrosis in patients taking BP 14,16. Ruggiero et al. 22 report that gnathic bones have a higher incidence of OAB when compared to other bones, which may be favored by the contact between bone tissue and oral cavity so that any injury or trauma (dental extractions, trauma caused by prostheses, installation of implants) or periodontal disease may allow contact of microorganisms with the bone tissue, triggering infectious processes, particularly colonies of Actinomyces spp. 12,22.
From 40 to 86%, bone necrosis occurs due extractions, since bone remodeling is very important to the healing process after tooth extraction. Thus, postoperative alveolar bone exposure, which is usually of short duration in healthy patients, not regresses in patients treated for BP, becoming infected and progressing to necrosis 20,26. Five of our patients had bone exposition after tooth extractions and two after dental implants, and none of them had previous information on the risks of oral invasive procedures related to BP medication. Neither the physician, nor the oral surgeon informed the patients about the effects of therapy on bone repair.
Although OAB is associated to traumatic injuries in most cases, 30% of patients may have spontaneous exposure, especially in areas easily damaged and covered with very thin mucosa 3,4.
BP toxicity affects also epithelial cells of the soft tissues and can play a role in OAB etiology as it contributes to continued exposure of the underlying bone with subsequent progression to infection and bone necrosis 10. Prosthesis maladaptation caused bone exposure in two of our patients, showing the importance of adaptation verification and necessary adjustments on frequent follow-up.
Main concern of patients attended in our service was bone exposition and medical referral. The lack of information about BP effects lead to inadequate treatment and eventually to bone exposition. OAB can be prevented with appropriate follow-up.
Initially OAB is not radiographically detected and patient may be asymptomatic 10,16. In more advanced cases, osteosclerosis, persistent unhealed alveoli, bone sequestration and lacunar osteolysis may appear 10,26. Often patients remain asymptomatic until there is a secondary infection by bone exposure, reporting as initial complaint, the presence of sudden intra oral discomfort, and sense of roughness that progresses to soft tissue trauma with exposure of necrotic bone. At this late stage, patients may complain of severe pain and paresthesia for nerve compression 15,16.
Osteonecrosis is a progressive condition, which if neglected, may result in extensive areas of exposed bone, dehiscence, bucconasal and sinusal communications, fistulas and even pathological fractures 15-17.
Treatment of patients with OAB is difficult and therapeutic options are scarce. Therapeutic is proposed according to clinical signs and symptoms during examination 4. In the presence of small and painless bone exposure, treatment is more conservative and mouthwashes with 0.12% chlorhexidine gluconate should be prescribed. If the patient has pain or evidence of infection, antiseptic mouthwash and systemic antibiotics must be prescribed, although there is controversy about the potential action of this drug due to vascular changes in bone tissue 4,10,25. When possible and appropriate, necrotic or infected bone sequestration should be removed, and, in severe cases of large exposures, marginal or segmental resections are indicated 4,16,19.
Patients with complete denture should be instructed to restrict use in order to reduce contact between prosthesis and exposed bone, in cases of prosthetic trauma 10. Hyperbaric oxygen therapy and laser therapy have been used as adjuvant treatments, but have inconclusive efficiency, requiring more studies 4,12,19.
Subramanian et al. 27 propose the use of teriparatide, a synthetic peptide that corresponds to parathormone (PTH), administered once a day, in low subcutaneous doses, for more than 24 months. This substance increases the osteoblast function by inhibiting its apoptosis, promoting osteoblast progenitor cells differentiation and stimulating the proliferation of these cells, expanding the number of osteoblasts precursors, which are actively involved in osteoblasts-osteoclasts relationship 27.
Mozzati et al. 17 suggest resection of necrotic bone and use of small autogenous grafts associated with platelet rich plasma (PRP), based on the assumption that the presence of growth factors, normally inhibited by BP, represents a surrogate stimulation to bone healing, turning it similarly to physiological.
Discontinuation of BP therapy to promote repair of necrotic bone tissues of the oral cavity has no scientific proof, but it can be discussed with the oncologist, considering the risks and benefits 15,16,25. Bisphosphonates have long half-life and treatment cessation may have a minimal effect considering medication already incorporated into bones. However, the antiangiogenic effect may be reduced, improving the healing of soft tissue overlying 16.
Thus, preventive actions become essential to establish adequate treatment for patients who will undergo BP therapy, and to plan specific treatment for patients who will develop OAB. All patients should be evaluated by a dentist prior to BP therapy 15,16, so that professional is well informed about treatment such as diagnosis, history of treatments and oral complications associated, expected treatment toxicity full blood examination, type of BP used, protocol of administration and expected time of therapy duration 16.
Furthermore, the dentist may establish a dental treatment based on the real needs of the patient. Oral hygiene orientation and elimination of all infected foci and potential sites of infection are performed to achieve good oral health, as well adaptation and guidance on the use of prostheses. Patients must be informed of drug effects on oral treatments, which can be performed and which should be avoided 10,16. Moreover, close follow-up are indispensable during and after treatment in order to detect early onset of avascular necrosis of the bone and start appropriate treatment 16.
Conclusion
Dentists should advise their patients about the use of BP and the implications for oral health and treatments. It is also necessary to perform an effective treatment prior to initiating therapy, to achieve good oral health, preventing further unwanted interventions. These patients must have periodic consultations for evaluation and early detection of OAB, for beginning of adequate treatment.
Therefore, patients undergoing to BP therapy must have a multidisciplinary attention to prevent possible complications that can be of difficult resolution.
References
1. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369-76. [ Links ]
2. Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and others malignancies. Oral Oncology. 2008;44:857-69.
3. Caldas RJ, Pontes JRM, Antunes HS. Osteonecrose dos maxilares induzida por bifosfonatos: relato de caso clínico. Rev Brasileira de Cancerologia. 2009;55(2):151-5.
4. Carvalho PSP, Santos HF, Duarte BG, Carvalho FA, Dias-Ribeiro E, Rocha JF. Principais aspectos da cirurgia bucomaxilofacial no paciente sob terapia com bifosfonatos. RFO. 2010;15(2):183-9.
5. Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296(2):235-42.
6 . Ebetino FH, Hogan AML, Sun S, Tsoumpra MK, Duan X, Triffitt JT et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone. 2011;49(1):20-33.
7. Hansen T, Kunkel M, Weber A, James Kirkpatrick C. Osteonecrosis of the jaws in patients treated with bisphosphonates –histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155-60.
8. Henneman ZJ, Nancollas GH, Ebetino FH, Russell RGG, Phipps RJ. Bisphosphonate bone affinity as assessed by inhibition of carbonated apatite dissolution in vitro. J Biomed Mater Res. 2008;85(4):993-1000.
9. Herbozo PJ, Briones DL, Ferres AJ, Torrealba RL. Severe spontaneous cases of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(8):1650-4.
10. Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenyl-tion: evidence from structure–activity relationships in J774 macrophages. J Bone Miner Res. 1998;13(11):166668-78.
11. Manfredi M, Merigo E, Guidotti R, Meleti M, Vescovi P. Bisphosphonate – related osteonecrosis of the jaws: a case series of 25 patients affected by osteoporosis. Int J oral Maxillofac Sur. 2011;40:277-84.
12. Martins MAT, Martins MD, Lascala CA, Curi MM, Migliorati CA, Tenis CA et al. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: A preliminary study. Oral Oncology. 2012;48:79-84.
13. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115-7.
14. Maurer P, Sandulescu T, Kriwalsky MS, Rashad A, Hollstein S, Stricker I et al. Biphosphonate-related osteonecrosis of the maxilla and sinusitis maxillaris. Int J Oral Maxillofac Sur. 2011;40:285-91.
15. McLeod MNH, Davies BJB, Brennan PA. Bisphosphonate osteonecrosis of the jaws; an increasing problem for the dental practitioner. British Dental Journal. 2007;203(11):641-4.
16. Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. O tratamento de pacientes com osteonecrose associada aos bifosfonatos. Uma tomada de posição da Associação Americana de Medicina Oral. JADA. 2006;6(3):5-16.
17. Mozzati M, Gallesio G, Arata V, Pol R, Scoletta M. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: a report of 32 cases. Oral Oncology. 2012;48:469-74.
18. Nancollas GH, Tang R, Phipps RJ, Henneman Z, Guld S, Wu W et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38(5):617-27.
19. Pérez SB, Barrero MV, Hernández MS, Knezevic M, Navarro JMC, Millares JR. Bisphosphonate-associated osteonecrosis of the jaw. A proposal for conservative treatment. Med Oral Patol Oral Cir Bucal. 2008;13(12):770-3.
20. Regev E, Lustmann J, Nashef R. Atraumatic teeth extraction in bisphosphonate-treated patients. J Oral Maxillofac Surg. 2008;6666(6):1157-61.
21. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12):2961-78.
22. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527-34.
23. Ruggiero S, Woo V, Mehrotra B, Fantasia J. Osteonecrosis of the jaws associated with the use of the bisphosphonates medications: a report of 60 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(2):196-7.
24. Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporosis Int. 2008;19(6):733-59.
25. Santos PSS, Gambirazi LM, Felix VB, Magalhães MHCG. Osteonecrose maxilar em pacientes portadores de doenças neoplásicas sob o uso de bifosfonatos. Rev Bras Hematol Hemoter. 2008;30(6):501-4.
26. Schubert M, Klatte I, Linek W, Müller B, Döring K, Eckelt U et al. The saxon bisphosphonate register – therapy and prevention of bisphosphonate-related osteonecrosis of the jaws. Oral Oncology. 2012;48:349-54.
27. Subramanian G, Cohen HV, Quek SYP. A model for the pathogenesis of bisphosphonate-associated osteonecrosis of the jaw and teriparatide's potential role in its resolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):744-53.
28. Tekin U, Tüz HH, Onder E, Ozkaynak O, Korkusuz P. Effects of alendronate on rate of distraction in rabbit mandibles. J Oral Maxillofac Surg. 2008;6666(10):2042-9.
29. Van Beek E, Löwik C, Que I, Papapoulos S. Dissociation of binding and antiresorptive properties of hydroxybisphosphonates by substitution of the hydroxyl with an amino group. J Bone Miner Res. 1996;11(10):1492-7.
30. Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;6666(4):625-31.
 Corresponding author:
Corresponding author:
Juliana Lucena Schussel
Rua Dr. Ovande do Amaral, n. 201
CEP 81520-060 – Curitiba – PR – Brasil
E-mail: juliana.schussel24@gmail.com
Received for publication: April 24, 2013
Accepted for publication: August 12, 2013













