Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.4 Joinville Out./Dez. 2013
Literature Review Article
Application of the finite element method in Dentistry
Máyra Andressa R. V. Piccioni I; Edson Alves Campos I; José Roberto Cury Saad I; Marcelo Ferrarezi de Andrade I; Marília Regalado Galvão I; Andrea Abi Rached I
I Department of Restorative Dentistry, School of Dentistry of Araraquara, São Paulo State University – Araraquara – SP – Brazil
ABSTRACT
Introduction: The finite element method (FEM) involves a series of computational procedures to calculate the stress in each element, which performs a model solution. Such a structural analysis allows the determination of stress resulting from external force, pressure, thermal change, and other factors. This method is extremely useful for indicating mechanical aspects of biomaterials and human tissues that can hardly be measured in vivo. The results obtained can then be studied using visualization software within the FEM environment to view a variety of parameters, and to fully identify implications of the analysis. Objective: An overview to show application of FEM in dentistry was undertaken. Literature review: This paper shows the basic concept, advances, advantages, limitations and applications of finite element method (FEM) in dentistry. Conclusion: It is extremely important to verify what the purpose of the study is in order to correctly apply FEM.
Keywords: finite element analysis; models; Dentistry.
Introduction
The oral cavity is a complex biomechanical system. Due to this complexity and limited access, most biomechanical research of the oral environment such as restorative dentistry, endodontics, orthodontics, prosthodontics and implantology has been performed in vitro. Mechanical tests have been used for determination of fracture resistance, behavior and mechanical properties of tooth structures and restorative materials, but these tests hardly provide information about internal behavior of the structures studied.
Deformation and stresses are generated when loads are applied to a structure. This is usual, and is how a structure performs its structural function. But if stresses become excessive and exceed the elastic limit, structural failure may result 40. These stresses cannot be directly measured and it is not easy to understand why and when a failure process is initiated in complex structures, and how we can optimize the strength and longevity of the components of the stomatognathic system. So, the application of engineering knowledge in dentistry with the use of computational techniques has helped to understand oral biomechanics aspects.
Finite Element Analysis (FEA) has been widely used through numerical analysis that has been successfully applied in many engineering and bioengineering areas since the 1960s. The finite element method (FEM) is a numerical procedure for analyzing structures. Usually, the problem addressed is too complicated to be satisfactorily solved by classic analytical methods. The problem may concern stress analysis, heat conduction, large deformations, fracture propagation or any of several other areas.
FEA is based obtaining a solution to a complex physical problem by dividing the problem domain into a collection of much smaller and simpler domains in which the field variables can be interpolated with the use of shape functions 20. The structure is discretized into so called "elements" connected through nodes. When choosing tFhe appropriate mathematical model, element type and degree of discretization are important to obtain accurate as well as time and cost effective solutions. Other advantages of this method compared with other research methodologies are the low operating costs, reduced time to carry out the investigation and information provision that cannot be obtained by experimental studies 40.
FEM has been applied in many studies of various areas of Dentistry. This can be attributed to mechanical engineering principles that are present in these specialties, favoring the use of finite element modeling.
The aim of this article is to show the use of FEM in the various areas of Dentistry.
Literature review
Prosthodontics
Different test parameters and standards are used in the experimental studies, which might be the cause of the controversy surrounding the issue of fracture resistance of teeth restored with endodontic posts. The biomechanical conditions that lead to fracture are characterized by the stress state in a tooth, which can be assessed by FEA.
FEM has been shown to be a useful tool when investigating complex systems that are difficult to standardize during in vitro and in vivo studies. It has been used to evaluate the influence of the type of material (metal, carbon, glass fiber and zirconia ceramic) and the external configuration of the dowel (smooth and serrated) on the stress distribution of teeth restored with varying dowel systems 45.
Some studies 6,36,45,47 found that the use of glass fiber dowel resulted in lower stresses "inside the root" than did zirconia ceramic, carbon fiber or metal dowel, but these studies failed to mention if these stresses were found in the dentin or within the dowel itself. Thus, there may be no risk of root fracture in this area. These studies showed the same conclusion, but it is interesting to note that material properties, loading conditions and simulated boundaries had some differences between researchers. The elastic constants used in the calculations were obtained from the literature; however there were variations between them. Moreover, different loading conditions were considered and many researchers considered glass fiber (GF) post to be transversally isotropic. Accuracy of the model was checked using convergence tests by Spazzin et al. 47. The results were presented in terms of von Mises criteria by researchers.
It has been reported that a cervical ferrule preparation creates a positive effect in terms of reducing stress concentration in endodontically treated teeth. Eraslan et al. 16 showed that the use of a ferrule in endodontically treated teeth restored with an all-ceramic post-and-core reduces the values of von Mises stresses on the tooth-restoration complex. Stress levels were higher for the rigid zirconium oxide ceramic post system than for fiber posts, at the dentin wall and within the post. Materials used in study were assumed as homogenous and isotropic. The elastic properties of the materials (Young's modulus [E] and Poisson's ratio [μ]) were obtained from the literature. Results were presented by considering von Mises criteria.
Studies 4,6 have emphasized the effect of the elastic modulus of the post material on stresses transferred to tooth structures showing that increasing the elastic modulus of the post causes decreased dentin stress. However, Boschian et al. 8 reported that post materials that have an elastic modulus higher than dentin are capable of causing dangerous and non-homogenous stresses in root dentin. Therefore, it is commonly accepted that a better performance is achieved if the stiffness of the post material is similar to that of dentin. Additionally, Silva et al. 45 concluded that the post material seems to be a more relevant factor on the stress distribution of endodontically treated teeth restored with a post than the post's external configuration.
Cements, such as glass ionomer, resin-modified glass ionomer, zinc-phosphate, and resin cement, have been used to fix dowels and cores with acceptable clinical results. Nevertheless, the difference in elastic modulus among dentin, intraradicular retainers, and cements could result in stress concentration at the restoration interface when the tooth is under function. Soares et al. 46 and Suzuki et al. 49 investigated the stress distribution in roots restored with different cements demonstrating that resin cement presented fracture resistance values that were significantly higher than the other cements. Moreover, Soares et al. 46 revealed that zinc–phosphate cement and conventional glass ionomer cement (GIC) produced higher stress concentration levels at the cement/dentin interface.
These findings were confirmed by Al-Omiri et al. 2, through a systematic review. They reported that the treatment of endodontically treated teeth using posts might be more successful if tooth structure loss is limited, a ferrule is obtained, a post with similar physical properties to natural dentin is used and the appropriate adhesive techniques for post luting and coronal restoration are used. Therefore, when the advantages and disadvantages of different luting systems and materials are considered, adhesively luted resin/fiber posts with composite cores appear to be the best luting technique currently available in terms of tooth fracture resistance and biomechanical behavior.
Restorative Dentistry
FEM has been used to analyze stresses generated in teeth and restorations. It has proven to be a useful tool for understanding tooth biomechanics and the biomimetic approach in restorative dentistry.
There is doubt whether a high or a low modulus of elasticity is preferable for composite restorations. Asmussen et al. 5 studied Class I and Class II resin composite restorations and the influence of the modulus of elasticity on stresses generated by occlusal loading. They concluded that resin composite occlusal restorations should have a high modulus of elasticity in order to reduce the risk of marginal deterioration. Yamamoto et al. 50 showed that materials with a higher modulus of elasticity were suitable as cavity base materials for posterior restorations. Asmussen et al. 5 obtained elastic constants of materials by literature and the restorations were loaded centrally with a force of 100 N in the axial while Yamamoto et al. 50 determined elastic modulus and Poisson's ratio by nanoindentation and a total oclusal load of 600 N was applied along the cusp.
Restorative procedures can make the tooth crown more deformable. Magne et al. 30 evaluated five models: natural tooth, MO and MOD cavities, MO and MOD endodontic access preparations and showed that the progressive loss of tooth structure (MO to MOD to endodontic access) translates into a progressive loss of cuspal stiffness. The natural tooth and the tooth with the MOD ceramic inlay presented the same behavior (100% recovery of cuspal stiffness). Boundary conditions, load protocol and configuration were chosen because they reproduce existing experiments. Material properties were based in literature. According to the authors the potential use of the model was demonstrated using nonlinear contact analysis to simulate occlusal loading. Cuspal widening was measured at different restorative steps and correlated with existing experimental data for model validation and optimization.
Campos et al. 9 and Belli et al. 7 investigated the use of ceramics and polymers in indirect restorations. They determined that ceramic seems to be a preferable material for restoring because its structure keeps the stress inside and, therefore, transfers less stress through the tooth structure. Mechanical properties, geometry, and thickness of the restorative material can directly influence the load distribution in a tooth/restoration complex and consequently the results.
Several studies 25,26,41,42 have suggested that the complex interaction caused by a non-functional distribution of occlusal loads, combined with poorly developed enamel and the demineralizing and weakening effects of erosive acids, may operate to produce non-carious cervical tooth loss. Ichim et al. 26 showed that the restorative materials (GIC and composite) currently used in non-carious cervical lesions are largely unsuitable in terms of resistance to fracture, suggesting that the elastic modulus of such a material should be in the range of 1 GPa. Whereas the location of the lesion and occlusal loading direction will affect the stress conditions, thus there will be many possibilities of model's type and it seems difficult to compare them. So, the ideal is to combine clinical observations and finite element modeling will be essential to determine the stress factor in the initiation and development of NCCL.
Restorations mean a change in the biomechanical balance of natural teeth. Problems may occur when the restorations are submitted to stressing conditions. Stress can interfere with the adhesive interface, enamel, or dentin substrate. Therefore, the elastic properties of the resin-dentin interface might have an important function for stable dentin bonding and in the prevention of gap formation. Some studies 3,7,35,52 evaluated the relation between the hybrid layer and stress distribution, showing that the hybrid layer has a stress-absorbing property and an increase in thickness of the adhesive layer increased the absolute values of stress concentration. The material properties (Young's module (E) and Poisson coefficient (n)) were established from literature. All materials were considered homogenous, isotropic and linearly elastic using 2-dimensional FEA by Anchieta et al. 3. Already, Belli et al. 7 presented the results in terms of the von Mises stress values. Xavier et al. 52 considered 3-D models as more reliable than the 2D models for analyzing the shear and microshear bond strength tests.
Implantology
FEA has been extensively used to predict the biomechanical performance of various dental implant designs, as well as the effect of clinical factors on the success of implantation.
Genj et al. 21 reviewed the current status of FEA applications in implant dentistry and discussed findings from FEA studies. Those authors concluded that, to achieve more realistic models, advanced digital imaging techniques can be used to model bone geometry in greater detail; the anisotropic and non-homogenous nature of the material needs to be considered; and boundary conditions must be refined. In addition, stress distribution in the implant–prosthesis connection has been examined by FEA studies because of the incidence of clinical problems, such as gold and abutment screw failures and implant fracture. Design changes to avoid or reduce these prosthetic failures by improving the stress distribution of implant components were suggested.
In a finite element study on immediately loaded implants, Ding et al. 14 showed that the simulated masticatory force was better dissipated and the stress and strain around the implant neck was decreased when the implant diameter was increased. Several studies 1,13,29,31,32,43 using FEA have found that a higher risk of bone resorption occurs in the neck region of an implant. By using the FEM, the authors could compare the elastic modulus and deformation of different types of bone, which helps clinicians to understand the process of bone remodeling, for further improvements of their surgery techniques.
The biomechanical behavior of the implant threads plays an important role on the stresses at the implant-bone interface. Eraslan et al. 17 found that different implant thread forms did not affect the von Mises stress distributions in supporting bone structure, but produced different compressive stress intensities in the bone. Materials used in study were assumed as homogenous and isotropic. The elastic properties of the materials ([E] and [μ]) were determined from the literature. Chun et al. 12 found that the square thread shape filleted with a small radius was more effective on stress distribution than other dental implants used in the analyses.
Early loading of dental implants after placement is believed to be a major cause for premature implant failure. Dos Santos et al. 15 showed that the simulations with non-submerged implants showed higher values of stress concentration than those that were submerged. It was also demonstrated that soft liner materials presented better results than when the denture base was not relined. The height of the healing caps seems to have a direct influence on the stress distribution in the peri-implant bone during the healing period. Considering the values obtained in that study, the use of soft liners with submerged implants seems to be the most suitable method to be used during the osseointegration period. The data were evaluated using Maximum Principal Stress.
Orthodontics
FEM has proved to be a valid and reliable technique for evaluating the deformation and loading characteristics of complex structures following the application of orthodontic forces. Cattaneo et al. 10 indicated that, following the application of an orthodontics loading regime, the concept of resorption is caused by compression, and formation is caused by tension. Furthermore, the same authors 11 found that tension in the alveolar bone was far more predominant than compression.
Studies 33,34,38,39 developed finite element models to understand the interaction between the periodontal ligament (PDL) and tooth mobility. Jones et al. 27 validated a FEM model and discovered that the PDL is the main mediator of orthodontic tooth movement, revealing that PDL demonstrated an inicial elastic response followed by a visco-elastic phase when subjected to a continuous load and the materials properties of periodontal ligament were difficult to quantify. Qian et al. 39 conducted a study by means of a combined experimental and numerical approach, to investigate the full-field distributions of displacement, stress and strain, and their evolution with loading in the entire fresh periodontium under an externally applied force. They concluded that the non-linear and time-dependent viscoelasticity of the PDL enables the acquisition of a full picture of detailed, realistic stress/strain fields, and deformation patterns of the entire fresh periodontium.
Discussion
FEM usually consists of 3 principle steps: Pre-processing, processing and post-processing. The objective of Pre-processing is the constructing of the "model" that consists of the geometrical representation, the definition of the material properties and boundary conditions.
FEA can be performed using two-dimensional (2D) or three-dimensional (3D) models. The choice between these two depends mainly on the required accuracy and the applicability of general findings associated to final time and costs involved 37. There are advantages and limitations of both approaches (table I).
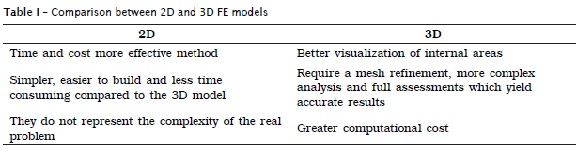
FEA models can follow different protocols, depending on the aim of the study. Models used to analyze laboratory test parameters usually have the simplest geometries and can be generated directly into the FEA software. Modeling of 2D and 3D biological structures may have to be performed with Computer Aided Design (CAD) or Bio-CAD software. Ideally, the teeth must be represented in 3-D.
FEM is performed with material properties that can be isotropic (same properties) or anisotropic (different properties along 3 axes: x, y, and z) 20. One of the difficulties associated with finite element models is to allocate appropriate physical properties to the different constituent parts of the tooth and restorative materials. The properties allocated to the materials under investigation are critical to the validity of FEAs, since each element is assigned specific values that affect the results.
Different researchers have used different physical characteristics of dental tissues, such as enamel and dentin. Wakabayashi et al. 51 considered enamel to be an isotropic material in which properties are similar in all directions, but when enamel is considered to be anisotropic, the tooth seems to be better able to cope with loading. Not only are the resultant stresses of lower magnitude, but they are also preferably transferred into dentin, which tolerates tensile stress better than the enamel 48. Already, some heterogeneity and anisotropy was demonstrated for dentin. However, the stiffness response seems to be only mildly anisotropic 22. Therefore, dentin properties are usually assumed to be isotropic.
The knowledge of the properties of the material as Elastic modulus, Poisson's ratio (strain in the lateral direction to that in the axial direction when an object is subjected to tensile loading), and shear modulus is required in FEM. The analysis is performed as linear static analysis or non-linear analysis depending on the allocation of appropriate physical characteristics to the different parts of the structure 20.
Most of the previously discussed studies employed linear static models. Linear analyses are valid if the structure exhibits a linear stress–strain relationship up to a stress level known as the proportional limit, and all the volumes are bonded as one unit. However, the validity of a linear static analysis may be questionable when the study objectives are to explore more realistic situations that are usually encountered in the intraoral environment. Realistic testing situations will give rise to nonlinearities, which can be grouped into the following principal categories: (1) material nonlinearities that cause the stiffness of a structure to change with different load levels; (2) changing interrelation of objects that is commonly seen in tooth-to-tooth and material-to-tissue contacts; and (3) geometric nonlinearities that are characterized by large deformations and/or rotations, and are occasionally seen in dental materials such as "dental wires".
Nonlinear analysis has become an increasingly powerful approach to predict the stress and strain within structures in a realistic situation that cannot be solved by using the linear static model. The application of the nonlinear FEM in dentistry seems to be interesting, for example in: nonlinear simulation of periodontal ligament properties, plastic and viscoelastic behaviors in materials, tooth-to-tooth contact analyses, contact analyses in implant structures and interfacial stress in restorations. However, there are difficulties, for example: the dynamic behavior of the PDL is an aspect to be considered, and simulation using nonlinear analysis would be more realistic 39. But, due to its complex structure; the exact mechanical properties of PDL must still be considered poorly understood. Thus, the incorrect use or questionable nonlinear mechanical properties in FEA may be more obscuring than a well defined and understood simplification. Some simplifications and assumptions are common in FEA. These practices are allowed, but their impact on the conclusions should be carefully taken into account 40.
In FEA the whole domain is divided into smaller elements. The collection and distribution of these elements is called a mesh. Elements are interconnected by nodes, which are thus the only points though which elements interact with each other. There are many different types of elements. One of the differences can be their basic shape, such as triangular, tetrahedral, hexahedral etc. 20. The basic concept is to use hex (linear) elements in critical areas (high stress locations) and tetra meshing (parabolic) in general areas (areas away from critical areas). The creation of mesh can be automatic or manual mesh generation. Usually, first auto-meshing is made, but after the manual controls are realized. A more accurate solution is obtained when a mesh is made finer, but the element count increases and the computation time also increase. One method to perform a converge study which consists in the creation and analysis of multiple mesh distributions with increasing number of elements or refinements 20.
The boundary conditions define the external influences on a modeled structure, usually loading and constraints. Restrictions can be summarized as the imposition of displacements and rotations on a finite element model, which can be either null or have fixed values or rates. These restrictions concern three rotations (around X, Y, Z-axes) and three translations (in X, Y, Z-directions). Boundary conditions are usually applied to nodes, where in a 3D model each free node has 6 degrees of freedom (3 translations and 3 rotations) 20. The application of loads in a FEA model must also represent the external loading situations to which the modeled structure is subjected. These loads can be tensile, compressive, shear, etc. It is important to note that a point of load application may result in high stress concentrations around the loaded nodes, creating unrealistic stress concentrations.
Nevertheless, there is difficulty due to the elaborate models used in dentistry, depending on the different shapes on the tooth to be analyzed and the difficulty involved in obtaining the mechanical properties of the tooth's constituent materials. Furthermore, little is known about the interfaces between these materials and their degree of influence on the mechanical behavior of the tooth as a whole. The properties and boundary conditions in the dentistry are dealing with complex and often little understood, therefore requiring assumptions and simplifications in the modeling of the stress-strain responses. The complexity of a FEA can differ depending on the modeled structure and research question. Furthermore, large anatomical variability precludes conclusions based on unique solutions.
Processing is the step in which the computer software does the work of calculation and Post-processing consists viewing the results, verifications, and conclusions. In FEA, the stress distribution analysis can be recorded by von Mises criteria or maximum principal stress. The stress analysis of von Mises does not have appropriate failure criterion for brittle materials. Therefore, maximum principal stress can be adopted to analyze the results.
Conclusion
It is well established that numerical analysis methods are of paramount importance, not only in aerospace, civil engineering and the automotive industry, but also in health care. FEM has proven itself an extremely powerful tool in addressing many biomedical problems that are challenging for more conventional methods because of structural and material complexity. The modeling and simulation step saves time and money for conducting the live experiment or clinical trial. But, the most powerful application of FEA is when it is conducted in combination with laboratory studies. FEA still needs laboratory validation to prove its results.
Therefore, this tool has been successfully employed in various areas of dentistry, but it is extremely important to verify what the purpose of the study is in order to correctly apply FEM. More studies are required to use appropriate models.
References
1. Almeida DAF, Pellizzer EP, Verri FR, Santiago Jr JF, Carvalho SP. Influence of morse taper and external hexagon connections on bone stresses around tilted dental implants. Three-dimensional finite element method with statistical analysis. J Periodontol. 2013 May;20. [ Links ]
2. Al-Omiri MK, Mahmoud AA, Rayyan MR, Abu-Hammad O. Fracture resistance of teeth restored with post-retained restorations: an overview. J Endod. 2010;36(9):1439-49.
3. Anchieta RB, Rocha EP, Ko CC, Sundfeld RH, Martin Junior M, Archangelo CM. Localized mechanics of dentin self-etching adhesive system. J Appl Oral Sci. 2007;15(6):321-6.
4. Asmussen E, Peutzfeldt A, Sahafi A. Finite element analysis of stresses in endodontically treated, dowel-restored teeth. J Prosthet Dent. 2005;94(4):321-9.
5. Asmussen E, Peutzfeldt A. Class I and Class II restorations of resin composite: an FE analysis of the influence of modulus of elasticity on stresses generated by occlusal loading. Dent Mater. 2008;24(5):600-5.
6 . Barjau-Escribano A, Sancho-Bru JL, Forner-Navarro L, Rodríguez-Cervantes PJ, Pérez-Gónzález A, Sanchez-Marín FT. Influence of prefabricated post material on restored teeth: Fracture strength and stress distribution. Oper Dent. 2006;31(1):47-54.
7. Belli S, Eskitascioglu G, Eraslan O, Senawongse P, Tagami J. Effect of hybrid layer on stress distribution in a premolar tooth restored with composite or ceramic inlay: an FEM study. J Biomed Mater Res Part B: Appl Biomater. 2005;74(2):66665-8.
8. Boschian Pest L, Guidotti S, Pietrabissa R, Gagliani M. Stress distribution in a post-restored tooth using the three-dimensional finite element method. 66J Oral Rehabil. 2006;33(9):690-7.
9. Campos RE, Soares CJ, Quagliatto PS, Soares PV, Oliveira Junior OB, Santos-Filho PCF et al. In vitro study of fracture load and fracture pattern of ceramic crowns: a finite element and fractography analysis. J Prosthodont. 2011;20(6):447-55.
10. Cattaneo PM, Dalstra M, Melsen B. Strains in periodontal ligament and alveolar bone associated with orthodontic tooth movement analyzed by finite element. Orthod Craniofac Res. 2009;12(2):120-8.
11. Cattaneo PM, Dalstra M, Melsen B. The finite element method: a tool to study orthodontic tooth movement. J Dent Res. 2005;84(5):428-33.
12. Chun HJ, Cheong SY, Han JH, Heo SJ, Chung JP, Rhyu IC et al. Evaluation of design parameters of osseointegrated dental implants using finite element analysis. J Oral Rehabil. 2002;29:565-74.
13. Clelland NL, Ismail YH, Zaki HS, Pipko D. Three-dimensional finite element stress analysis in and around the screw-vent implant. Int J Oral Maxillofac Implants. 1991;6(4):391-8.
14. Ding X, Zhu XH, Liao SH, Zhang XH, Chen H. Implant–bone interface stress distribution in immediately loaded implants of different diameters: a three-dimensional finite element analysis. J Prosthodont. 2009;18(5):393-402.
15. Dos Santos MBF, Silva Neto JP da, Consani RLX, Mesquita MF. Three-dimensional finite element analysis of stress distribution in peri-implant bone with relined dentures and different heights of healing caps. J Oral Rehabil. 2011;38(9):691-6.
16. Eraslan O, Aykent F, Yücel MT, Akman S. The finite element analysis of the effect of ferrule height on stress distribution at post-and-core-restored all-ceramic anterior crowns. Clin Oral Invest. 2009;13(2):223-7.
17. Eraslan O, Inan O. The effect of thread design on stress distribution in a solid screw implant: a 3D finite element analysis. Clin Oral Invest. 2010;14(4):411-6.
18. Farah JW, Craig RG. Distribution of stresses in porcelain fused to metal and porcelain jacket crowns. J Dent Res. 1975;54(2):255-61.
19. Fernandes A, Dessai G. Factors affecting the fracture resistance of post-core reconstructed teeth: a review. Int J Prosthod. 2001;14(4):355-63.
20. Gallagher RH. Introduction. In: Gallagher RH. Finite element analysis: fundamentals. 4. ed. Englewood Cliffs: Prentice-Hall; 1975. cap. 1, p. 1-19.
21. Geng JP, Tan KBC, Liu GR. Application of finite element analysis in implant dentistry: A review of the literature. J Prosthet Dent. 2001;85(6):585-98.
22. Huo B. An inhomogeneous and anisotropic constitutive model of human dentin. J Biomech. 2005;38(3):587-94.
23. Hsu ML, Chen CS, Chen BJ, Huang HH, Chang CL. Effects of post materials and length on the stress distribution of endodontically treated maxillary central incisors: a 3D finite element analysis. J Oral Rehabil. 2009;36(11):821-30.
24. Ichikawa T, Kanitani H, Wigianto R, Kawamoto N, Matsumoto N. Influence of bone quality on the stress distribution. An in vitro experiment. Clin Oral Implants Res. 1997;8(1):18-22.
25. Ichim IP, Li Q, Loughran J, Swain MV, Kieser JA. Restoration of non-carious cervical lesions Part I. Modelling of restorative fracture. Dent Mater. 2007;23(12):1553-61.
26. Ichim IP, Schmidlin PR, Li Q, Kieser JA, Swain MV. Restoration of non-carious cervical lesions Part II. Restorative material selection to minimise fracture. Dent Mater. 2007;23(12):1562-69.
27. Jones ML, Hickman J, Middleton J, Volp C. A validated finite element method study of orthodontic tooth movement in the human subject. J Orthod. 2001;28(1):29-38.
28. Loney W. Three-dimensional photoelastic stress analysis of the ferrule effect in cast post and core. J Prosthet Dent. 1990;63(5):506-12.
29. Lozada JL, Abbate MF, Pizzarello FA, James RA. Comparative three-dimensional analysis of two finite-element endosseous implant designs. J Oral Implantol. 1994;20(4):315-21.
30. Magne P. Efficient 3D finite element analysis of dental restorative procedures using micro-CT data. Dent Mater. 2007;23(5):539-48.
31. Meijer HJ, Starmans FJM, Steen WHA, Bosman F. Loading conditions of endosseous implants in an edentulous human mandible: a three-dimensional, finite-element study. J Oral Rehabil. 1996;23(11):757-63.
32. Meijer HJ, Kuiper JH, Starmans FJM, Bosman F. Stress distribution around dental implants: influence of superstructure, length of implants, and height of mandible. J Prosthet Dent. 1992;68(1):96-102.
33. Middleton J, Jones M, Wilson A. The role of the periodontal ligament in bone modeling: the initial development of a time-dependent finite element model. Am J Orthod Dentofacial Orthop. 1996;109(2):155-62.
34. Natali AN, Pavan PG, Scarpa C. Numerical analysis of tooth mobility: formulation of a non-linear constitutive law for the periodontal ligament. Dent Mater. 2004;20(7):623-9.
35. Neves AA, Coutinho E, Poitevin A, Sloten JVd, Meerbeek BV, Oosterwyck HV. Influence of joint component mechanical properties and adhesive layer thickness on stress distribution in micro-tensile bond strength specimens. Dent Mater. 2009;25(1):4-12.
36. Pegoretti A, Fambri L, Zappini G, Bianchetti M. Finite element analysis of a glass fibre reinforced composite endodontic post. Biomaterials. 2002;23(13):266667-82.
37. Poiate IA, Vasconcellos AB, Mori M, Poiate Jr. E. 2D and 3D finite element analysis of central incisor generated by computerized tomography. Comput Methods Programs Biomed. 2011;104:292-9.
38. Provatidis CG. A comparative FEM-study of tooth mobility using isotropic and anisotropic models of the periodontal ligament. Med Eng Phys. 2000;22(5):359-70.
39. Qian L, Todo M, Morita Y, Matsushita Y, Koyano K. Deformation analysis of the periodontium considering the viscoelasticity of the periodontal ligament. Dent Mater. 2009;25(10):1285-92.
40. Raposo LHA, Armstrong SR, Maia R, Qian F, Geraldeli S, Soares CJ. Effect of specimen gripping device, geometry and fixation method on microtensile bond strength, failure mode and stress distribution: Laboratory and finite element analyses. Den Mater. 2012;28:e50-e62.
41. Rees JS. The effect of variation in occlusal loading on the development of abfraction lesions: a finite element study. J Oral Rehabil. 2002;29(2):188-93.
42. Rees JS, Hammadeh M. Undermining of enamel as a mechanism of abfraction lesion formation: a finite element study. Eur J Oral Sci. 2004;112(4):347-52.
43. Rieger MR, Mayberry M, Brose MO. Finite element analysis of six endosseous implants. J Prosthet Dent. 1990;63(6):671-6.
44. Schimitter M, Rammelsberg P, Lenz J, Scheuber S, Schweizerhof K, Rues S. Teeth restored using fiber-reinforced posts: in vitro fracture tests and finite element analysis. Acta Biomater. 2010;6(9):3747-54.
45. Silva NR, Castro CG, Santos-Filho PCF, Silva GR, Campos RE, Soares PV et al. Influence of different post design and composition on stress distribution in maxillary central incisor: Finite element analysis. Indian J Dent Res. 2009;20(2):153-8.
46. Soares CJ, Raposo LHA, Soares PV, Santos-Filho PCF, Menezes MS, Soares PBF et al. Effect of Different cements on the biomechanical behavior of teeth restored with cast dowel-and-cores-in vitro and FEA analysis. J Prosthodont. 2010;19(2):130-7.
47. Spazzin AO, Galafassi D, Meira-Júnior AD, Braz R, Garbin CA. Influence of post and resin cement on stress distribution of maxillary central incisors restored with direct resin composite. Oper Dent. 2009;34(2):223-9.
48. Spears IR, van Noort R, Crompton RH, Cardew GE, Howard IC. The effects of enamel anisotropy on the distribution of stress in a tooth. J Dent Res. 1993;72:1526-31.
49. Suzuki C, Miura H, Okada D, Komada W. Investigation of stress distribution in roots restored with different crown materials and luting agents. Dent Mater J. 2008;27(2):229-36.
50. Yamamoto T, Takeishi S, Momo Y. Finite element stress analysis of indirect restorations prepared in cavity bases. Dent Mater J. 2007;26(2):274-9.
51. Wakabayashi N, Ona M, Suzuki T, Igarashi Y. Nonlinear finite element analysis: advances and challenges in dental applications. J Dent. 2008;36:463-71.
52. Xavier TA, Ballester RY. A comparison between the capacity of 2D and 3D finite element models in analyzing the stress distribution in shear and microshear bond strength tests. J Res Dent. 2013;1(1):41-54.
 Corresponding author:
Corresponding author:
Máyra Andressa R. V. Piccioni
Rua Humaitá, n. 1.680 – Centro
CEP 14801-903 – Araraquara – SP – Brasil
E-mail: mayraandressa@ig.com.br
Received for publication: May 9, 2013
Accepted for publication: June 27, 2013













