Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.1 Joinville Jan./Mar. 2014
ORIGINAL RESEARCH ARTICLE
Comparative evaluation of pH and solubility of MTA Fillapex® endodontic sealer
Meiryelen Silva FingerI; Gislaine FaraoniI; Michel do Carmo MassonI; Rogério Aparecido Minini dos SantosI; Ana Claudia Baladelli Silva CimardiI; Fausto Rodrigo VictorinoI
I Dentistry Course, University Center of Maringá – Maringá – PR – Brazil
ABSTRACT
Introduction and Objective: To evaluate pH and solubility of MTA Fillapex® cement. Material and methods: Patients were divided into four groups: GI (MTA Fillapex®), GII (Sealer 26®), GIII (Sealapex®) and GIV (AH Plus®). Samples of each group with 10 mm in diameter and 2 mm in height were immersed into water at neutral pH and kept at 37ºC. After 3 hours, the first pH measurement was carried out and repeated at 24 hour intervals for seven days. The data were submitted to Anova (p < 0.05). To analyze the solubility, specimens with 20 mm in diameter and 1.5 mm in thickness were weighed after the setting time, and maintained in distilled water at 37ºC for seven days. After this period, the specimens were again weighed. The difference between their weighs represents the mass loss. The data were subjected to Student's t test for paired samples (p < 0.05). Results: GI and GIII showed pH increase at the first 24 hours, with a significant reduction compared with the other cements. GIV was the smallest mass loss, followed by GII, GIII and GI. Conclusion: MTA Fillapex® has higher solubility than that of resin cements, but its pH remained above 10 for seven days.
Keywords: pH; solubility; root canal obturation.
Introduction
The main goal of endodontic filling is to obtain the sealing of root canal system which favors the process of periapical process after endodontic therapy and prevents either coronal or apical marginal leakage. The improper sealing may result in the movement of fluids towards the cement gaps which may lead to periapical inflammatory reaction, compromising endodontic success 12.
Antimicrobial activity is also an important requirement of endodontic sealers and it is directly related to their releasing of hydroxyl ions leading to pH increasing and to the creation of an unfavorable environment for bacterial survivor 10.
Currently, it has been available in dental market many sealers with different formulations, and consequently different physical and chemical propert ies 16. Endodont ic cements can be classified into: resin-, zinc oxide and eugenol-, calcium hydroxide-, and glass ionomer cementbased sealers.
Mineral trioxide aggregate (MTA) was initially introduced in Endodontics for the sealing of root and retrofilling perforations because of its favorable physical, chemical, and biological properties 2,13. Notwithstanding, to be used as endodontic sealer, its formulation has to be upgraded to improve its flowing, setting time and bond strength 5.
MTA Fillapex® (Angelus, Londrina, PR, Brazil) is a MTA-based endodontic sealer currently launched in Brazilian dental market and little studies on its physical-chemical properties have been conducted. Therefore, the aim of this study was to evaluate pH and solubility of MTA Fillapex® and to compare its results with those of other endodontic sealers that have been used in clinical practice (Sealer 26®, Sealapex® and AH Plus®).
Material and methods
The sealers were divided into four groups: group I – MTA Fillapex® (Angelus, Londrina, PR, Brazil), group II – Sealer 26® (Dentsply, Petrópolis, RJ, Brazil), group III – Sealapex® (SybronKerr, Washington, USA) and group IV – AH Plus® (Dentsply, DeTrey, Konstanz, Germany).
pH test
To construct the samples, poly(vinyl chloride) (PVC) rings with 10 mm in diameter and 2 mm in height were employed. The rings were placed onto a thin cellophane sheet supported by a glass plate and filled with the sealers mixed according to each manufacturer's instructions. Just after that, a nylon thread with about 0.5 mm in diameter was inserted into the material mass and another cellophane sheet and glass plate were placed onto the rings filled with the sealers. A mass of 100 g was placed over this set. The samples were kept in an environment with temperature of 37°C for up to three times the setting time of each sealer. Six samples were constructed for each group.
Elapsed that time, the samples were immersed into flasks with 40 mm in diameter filled with 50 ml of distilled and deionized water (milli-Q type) whose pH was previously measured to prove its neutrality.
The flasks were carefully closed to prevent that the samples were in contact with the flasks' walls. All sets were maintained in an incubator at 37°C, and after 3 hours, the first pH measurement was performed. The following measurements were executed at 24 hour intervals for one week. At each evaluation period, the specimens were carefully removed, evaluated and again maintained into the same flasks, but with new water.
The pH measurement was performed with the aid of a pH meter (W3b – BEL Engineering, Piracicaba, SP, Brazil) previously calibrated with solutions of known pH: 4 and 7. Data found were submitted to ANO VA and Tukey test with level of significance of 5%.
Solubility test
PVC rings with 20 mm in internal diameter and 5 mm in thickness were employed. The rings were placed onto a thin cellophane sheet supported by a glass plate and filled with the sealers mixed according to each manufacturer's instructions. Just after that, a nylon thread with about 0.5 mm in diameter was inserted into the material mass and another cellophane sheet and glass plate were placed onto the samples filled with the sealer. A mass of 100 g was placed over this set. The samples were kept in an environment with temperature of 37°C for up to three times the setting time of each sealer. After that, the samples were removed from the rings.
Each sample was weighed in a precision scale (Ohaus Adventurer® – Toledo do Brasil, São Bernardo do Campo, SP, Brazil) and suspended through the nylon thread inside a large opening flask containing 50 ml of milli-Q ultrapure water. The ring was then placed into it without contacting the flasks' walls. These samples were maintained into an incubator at 37°C for seven days.
Elapsed that period, the samples were removed from the flask and washed in deionized water to remove possible residues. Then, the samples were placed into a desiccator for 24 hours for new weighing. The difference between the first and the second weighing represents the mass loss for each one of the specimens.
Data were analyzed by Student's t test for paired samples because it enables the analysis of results at two different periods, with level of significance of 5% (p < 0.05).
Results
pH test
Table I shows the pH mean values according to the experimental period for each sealer tested. ANO VA exhibited significant differences in pH among the periods tested for the same sealer; also, there were differences in pH among sealers at the same period (p < 0.05).
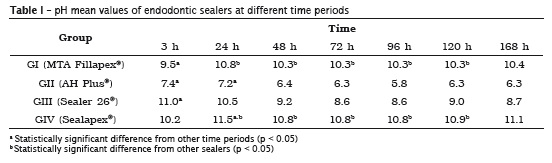
MTA Fillapex® and Sealapex® sealers exhibited a similar behavior with pH increasing of the solution at the first 24 hours, but with statistically significant reduction when compared with the other sealers. AH Plus® and Sealer26® also had a similar behavior because, unlikely the other sealers, they showed a pH reduction at 24 hours.
Solubility test
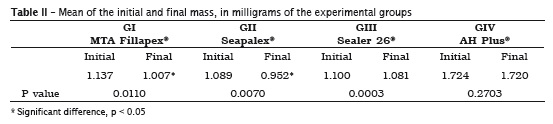
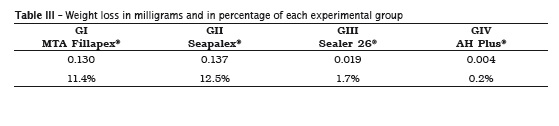
Table II displays the mean values of the initial and final weighs. Table III exhibits the weight loss in milligrams and in percentage.
Discussion
Calcium silicate-based MTA sealer was firstly introduced in Endodontics by Lee et al. 15 in 1993 as retrofilling material and for the repairing of perforations 19. Posteriorly, it has been indicated for pulp capping, apexification, and repairing of root resorption because of its favorable physical, chemical and biological properties 2,13. Its composition was based on hydrophilic particles that set in the presence of humidity. The hydration of the powder results in a colloidal gel that solidifies becoming a hard structure. MTA leads to an ideal physical sealing, being insoluble even in the presence of blood 3.
Most of the filling materials tend to contract moving away from the canal's wall, creating a gap for which the contaminants may penetrate. MTA setting results in the hydration of the compounds of anhydrous mineral oxide to produce calcium silicate hydrate and phases of calcium hydroxide, which expands against the canal's walls, improving the sealing and decreasing the leakage 12.
In order to use MTA as endodontic sealer, it was necessary to adjust its formulation to improving its flowing, setting time and bond strength 5. Currently, a new MTA-based endodontic sealer was launched in the Brazilian dental market – namely MTA Fillapex® (Angelus Soluções Odontológicas, Londrina, PR, Brazil) –, whose composition, according to the manufacturer, is basically MTA, silicate resin, natural resin and bismuth oxide.
The use of materials providing high alkalinity favors the mineralization of hard tissues and stimulates the body reaction, which quickens the process of repairing, clinically evidenced by biological sealing 4, as well as providing a good antimicrobial activity 11. Antimicrobial activity is promoted by pH increasing resulting from the releasing of hydroxyl ions because the alkaline pH induces the loss of the integrity of the cytoplasmic membrane of the cells, promotes the inactivation of the enzymes involved in the cellular metabolism, and damages bacterial DNA 11.
The releasing of hydroxyl ions occurs by the interaction of the sealer with water 6. Therefore, in this present study, the samples were placed into distilled water (milli-Q type) so that hydroxyl ions were formed consequently leading to pH alteration; also the water was renewed at every analysis period as previously reported by other authors 7-9, aiming to evaluate whether the ions releasing would occur at the intervals between the periods assessed and to reproduce which happens in vivo: the constant changes of fluids and renovation of ionic concentrations 6.
In this present study, MTA Fillapex®, AH Plus®, Sealer 26® and Sealapex® sealers were evaluated at 3, 24, 48, 72, 96, 120 and 168 hours after their immersion into milli-Q water. There was an increasing in the pH value for MTA Fillapex® over time, beginning with a pH of 9.5 and reaching to 10.4 in the last measurement. The same behavior was observed for Sealapex® sealer, which exhibited an initial pH of 10.2, finishing with a pH of 11.1. Such behavior can be explained by the solubilization of the surface of the samples during the successive water changes through dilution into water so that the highest the dilution the highest the degree of ionization is, that is, the highest the pH of the solution 6.
The pH peak of MTA Fillapex® and Sealapex® sealers was observed at 24 hours: 10.8 and 11.5 respectively for MTA Fillapex® and Sealapex®. This behavior was also observed by Kuga et al. 14 for MTA Fillapex® sealer, with pH value of 9.39 at the first 24 hours. These authors compared the pH value of MTA Fillapex® with that of grey and white MTA. The tested sealer evidenced a satisfactory alkalinity, but smaller than those of other sealers at the evaluation period. Morgental 18 reported a pH value for MTA Fillapex® around 10, which also corroborates the results evidenced by this present study. The highest pH values found in this present study were for MTA Fillapex® and Sealapex®, which were closer to the values established by McHugh 17 either to inhibit or eliminate E. faecalis, which is capable of surviving in an alkaline pH that normally would inhibit other bacteria 17.
As a physical property of a material, the insolubility can greatly impact on endodontic treatment success rate. Moreover, endodontic sealers must have low solubility because the leaching of their components can generate undesirable biological effects on the surrounding tissues 21. The endodontic filling materials are designed to be kept inside root canals to promote an impermeable sealing at long term and to eliminate any communication route between oral cavity and periapical tissues. Consequently, the low solubility level for these materials is of extreme importance 3.
Scelza et al. 20 studied the physical-chemical properties of endodontic sealers and demonstrated that Sealer 26® was the one that solubilized the least, followed by AH Plus®; unlikely, Sealapex® showed the highest solubilization. Contrary to this result, this present study demonstrated that AH Plus® sealer showed the smallest mass loss during the solubility test without statistically significant difference between the initial and final weighing, followed by Sealer 26®, which exhibited a mean mass loss of 1.7%.
Borges et al. 3 described that AH Plus® and MTA® Angelus sealer demonstrated to be soluble within the recommended range, while MTA Fillapex® and Sealapex® sealers exhibited values higher than those recommended by the American National Standards Institute / American Dental Association 1, results that corroborate with the findings of this present study, in which Sealapex sealer presented a higher level of mass loss, reaching 12.5%, while MTA Fillapex® reached 11.4%.
It is clear then that the most soluble sealers are composed by calcium hydroxide and because of this they show the highest pH values. Further studies on whether the releasing of calcium ions and pH increasing of MTA Fillapex® sealer would compensate its sealing capacity are necessary because these latter has been previously well presented by MTA used in retrofilling obturations.
Conclusion
It seems fair to say that the results found in this study corroborates those of the literature, in which MTA Fillapex® sealer showed high solubility when compared with that of other resin sealers, above 3%, within the range recommended by the American National Standards Institute / American Dental Association. However, its pH was kept above 10 for up to seven days. Also, further studies on the evaluation of its sealing capacity and antimicrobial activity are necessary.
Acknowledges
The authors thank to the University Center of Maringá by the granting of the Project of Scientific Initiation and to Dentsply of Brazil and Angelus Industry of Dental Products by providing the endodontic sealers.
References
1. American National Standards Institute / American Dental Association. Specification n. 57 for endodontic filling materials. Chicago: ADA; 2000. [ Links ]
2. Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35:777-90.
3. Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De-Deus G, Miranda CES et al. Changes in the surface of four calcium silicatecontaining endodontic materials and epoxy resinbased sealer after a solubility test. Int Endod J. 2012;45:419-28.
4. Broon NJ, Bramante CM, Assis GF, Bortoluzzi EA, Bernardineli N, Moraes IG et al. Healing of root perforations treated with mineral trioxide aggregate (MTA) and Portland cement. J Appl Oral Sci. 2006;14:305-11.
5. Camilleri J. Evaluation of selected properties of mineral trioxide aggregate sealer cement. J Endod. 2009;35:1412-7.
6. Carneiro D, Barbosa SV. Avaliação do pH dos cimentos endodônticos e considerações clínicas. Robrac. 1998;7:6-10.
7. Duarte MAH, Demarchi ACCO, Yamashita JC, Kuga MC, Fraga SC. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:345-7.
8. Duarte MAH, Martins CS, Demarchi ACOC, de Godoy LF, Kuga MC, Yamashita JC. Calcium and hydroxide release from different pulp-capping materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:66-9.
9. Eldeniz AU, Erdemir A, Kurtoglu F, Esener T. Evaluation of pH and calcium ion release of Acroseal sealer in comparison with Apexit and Sealapex sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:86-91.
10. Estrela C. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealepex and Dycal. Braz Dent J. 2000;11:3-9.
11. Estrela C, Sydney GB, Bammann LL, Felippe Júnior O. Mechanism of action of calcium and hidroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995;6:85-90.
12. Gomes-Filho JE, Moreira JV, Watanabe S, Lodi CS, Cintra LT, Dezan Jr. E et al. Sealability of MTA and calcium hydroxide-containing sealers. J Appl Oral Sci. 2012;20:347-51.
13. Gomes-Filho JE, Watanabe S, Estrada Bernabe PF, Costa MTM. A mineral trioxide aggregate sealer simulated mineralization. J Endod. 2009;35:256-60.
14. Kuga MC, Campos EA, Viscardi PH, Carrilho PZ, Xaviér FC, Silvestre NP. Hydrogen ion and calcium releasing of MTA Fillapex® and MTA-based formulations. RSBO. 2011;8:271-6.
15. Lee SJ, Monsef M, Torabinejad M. Sealing ability of mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19:541-4.
16. Leonardi DP, Battisti JC, Klimiont DT, Tomazinho PH, Baratto-Filho F, Haragushiku GA et al. Avaliação in vitro da ação antimicrobiana de alguns cimentos endodônt icos. RSBO. 2009;6:367-73.
17. Mchugh CP. pH required to kill Enterococcus faecalis in vitro. J Endod. 2004;30:218-9.
18. Morgental RD. Antibacterial activity of two MTA-based root canal sealers. Int Endod J. 2011;44:1128-33.
19. Santos AD, Moraes JC, Araújo EB, Yukimitu K, Valério Filho WV. Physico-chemical properties of MTA and a novel experimental cement. Int Endod J. 2005;38:443-7.
20. Scelza MFZ, Scelza P, Costa RF, Câmara A. Estudo comparativo das propriedades de escoamento, solubilização e desintegração de alguns cimentos endodônticos. Pesq Bras Odontoped Clín Integr. 2006:6:243-7.
21. Schäfer E, Zandbiglari T. Solubility of rootcanal sealer in water and artificial saliva. Int Endod J. 2003;36:660-9.
22. Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root-end canal filling materials: effects of blood contamination. J Endod. 1994;20:159-63.
 Corresponding author:
Corresponding author:
Fausto Rodrigo Victorino
Rua Formosa, n. 489 – Centro
CEP 86990-000 – Marialva – PR – Brasil
E-mail: frvictorino@ig.com.br
Received for publication: June 20, 2013
Accepted for publication: November 14, 2013













