Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.1 Joinville Jan./Mar. 2014
ORIGINAL RESEARCH ARTICLE
Analysis of tensile strength of poly(lactic-coglycolic acid) (PLGA) membranes used for guided tissue regeneration
Bruno Gasparini Betiatto de SousaI; Gabrielle PedrottiI; Ana Paula SponchiadoI; Rafael Schlögel CunaliI; Águedo AragonesII; João Rodrigo SarotIII; João Cézar ZielakI; Bárbara Pick OrnaghiI; Moira Pedroso LeãoI
I School of Dentistry, Positivo University – Curitiba – PR – Brazil
II School of Dentistry, Federal University of Santa Catarina – Florianópolis – SC – Brazil
III School of Dentistry, Federal University of Paraná – Curitiba – PR – Brazil
ABSTRACT
Introduction: The challenge of restoring patient's function that presented some loss of an organ or tissue encourages the Tissue Engineering and Biotechnology to develop materials that promote bone regeneration. Poly(lactic-co-glycolic acid) (PLGA) copolymer is among of the most biomaterials used. Objective: To evaluate the tensile strength of PLGA membranes at different conditions of humidity and temperature. Material and methods: PLGA membranes were hourglass-shape cut and prepared at three different conditions of temperature and humidity (n = 10): (I) dry membrane at environment temperature of about 20ºC (control group), (II) moist membrane plasticized at 55ºC, (III) moist membrane plasticized at 55ºC, which subsequently underwent cooling. Subsequently, the membranes were subjected to tensile tests in a universal testing machine (DL-2000, EMIC) at 1.0 mm/min. Data was submitted to ANOVA and Tukey's test (p < 0.05). Results: Group I showed the highest tensile strength mean (16.7 ± 1.9a MPa, p = 0.0022). There was no statistically significant difference between the means of groups II (14.6 ± 1.4 MPab) and III (13.9 ± 1.7 MPab). Conclusion: The dried PLGA membranes showed higher tensile strength than the membranes that were only either plasticized or cooled.
Keywords: PLGA membranes; tensile strength test; guided tissue regeneration.
Introduction
The loss of bone tissue resulting from lesions or other damages impacts the patient's life. The reconstruction of such structures through synthetic materials many times does not return the function and aesthetics required, making this a clinical challenge. The use of autogenous bone grafting collected from the patient is efficient; however, there is the need of a second surgical site because of the donor area. The most common donor areas used in Dentistry for bone grafting are: the skull bone, chin, iliac crest, retromolar area, and the maxillary tuberosity 2,6. Consequently, this cause greater morbidity to patient, contraindicating the surgical procedure 8. Allogeneic (from individuals of the same species) and xenogeneic (from one species and transplanted to other species) grafting has the advantages of not necessitating another second surgical site; however, they have disadvantages such as incompatibility of the host, risk of disease transmission and greater chance of resorption and consequently loss of the bone gain 2,17.
Other procedures can be executed aiming to increase the bone volume, such as osteogenic distraction (surgical induction of the bone fracture and splitting into two fragments so that a new bone is formed between them), osteoinduction with growth factors and/or stem cells, osteoconduction by the use of substrates for cellular development (scaffolds) and guided tissue regeneration (GTR) with the aid of membranes 18. GTR is an alternative basically based on the installation of mechanical barriers to protect the area of neoformed tissue avoiding that other tissues, e.g., connective and clots, invade and jeopardize bone formation 10 (figure 1).
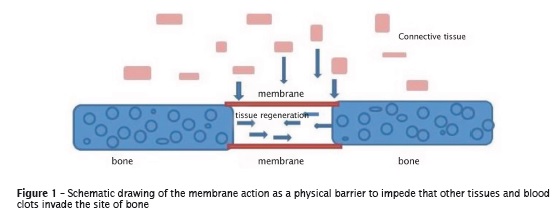
The membranes should be hard enough to maintain the space and support the tissues of the surgical area. Thus, it is needed that the constituting material of the membranes is malleable to provide the specific geometry for the functional reconstruction and hard to support external forces, such as those from mastication 7,18. Moreover, it is of great importance that they are totally biocompatible to not damage the surrounding tissues. Also, they should be porous, because it is through the pores that the fluids, nutrients, oxygen, and bioactive substances for cellular growth are changed. On the other hand, the diameter of the pores should be controlled. If they are very large, they can provide the leakage of fibroblasts, thus inhibiting the proliferation of stem cells and acting as route to bacteria 28.
The membranes can be constituted by either a single material or a combination of materials, such as the association of polymers with either collagen or hydroxyapatite. According to Pereira Neto et al. 15, still there is no consensus on which biomaterial would display the best performance in the tissue engineering. Commercially, resorbable and non-resorbable membranes have been found. Among the resorbable membranes, those constituted by polymers such as glycolic acid (PGA), polylactic acid (PLA) and poly(lactic-co-glycolic acid) (PLGA) are the most used. Their advantage is to not require a second surgical procedure decreasing the morbidity of the patient. One of the disadvantages is the possibility of collapse during degradation, resulting in the loss of the barrier function and consequently the invasion by other tissues on the regeneration area, leading to the procedure failure. Non-resorbable membranes are composed by titanium and polytetrafluoroethylene net. Although they require a second surgical procedure, they are stable and do not undergo collapse and act as a barrier until their removal, reducing the risk of complications 18.
Therefore, PLGA membranes are a good alternative for this purpose because they are biomaterials serving as physical support to guide tissue neoformation. Based on this information, the aim of this study was to evaluate the tensile strength of a resorbable PLGA membrane at different conditions of humidity and temperature.
Material and methods
Construction of the specimens
The membranes employed in this study were produced with PLGA copolymer and obtained by solvent evaporation technique 16. PLGA copolymer (Resomer, Evonik Ind., Essen, Germany), at 82:18 (m:m) ratio, was diluted in organic solvent dichloromethane formaldehyde (ChCl2, Synth – LabSynth, Diadema, Brazil). This solution was poured into rectangular moulds measuring 2.0 cm in width and 3.0 cm in length. After the solvent evaporation, the pieces were cut in rectangles (1.5 cm in width and 3.0 cm in length) to obtain samples with thickness ranging from 16 to 30 micrometers (figure 2). Next, the membranes were sterilized by gamma radiation (CBE, Cotia, Brazil).
To perform the tensile strength test, the membranes were cut in hourglass shape (5.0 mm in width at the central portion and 25.0 mm in length) with the aid of a guide of resin composite (figure 3).
Prior to the tensile strength tests, the samples were submitted to three different humidity and temperature conditions: (I) membranes dried at environmental temperature of about 20ºC (control group); (II) moist membranes and plasticized; (III) moist membranes and plasticized which were cooled subsequently. The membranes of groups II and III were plasticized for two minutes in 0.9% saline solution (Segmenta, Ribeirão Preto, Brazil), heated at constant temperature of 55ºC (model CRC-5AC2W, PolyScience, Niles, USA) (figure 4). The membranes of group III were cooled in 0.9% saline solution (Segmenta, Ribeirão Preto, Brazil) at 10ºC for 30 seconds.

Tensile strength test
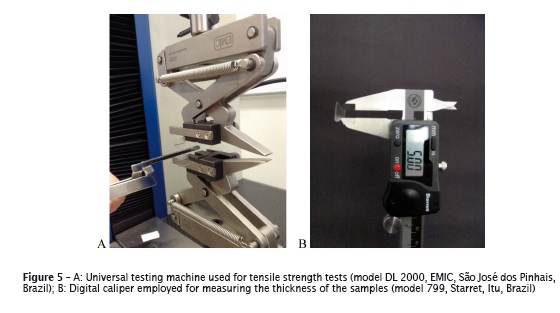
The tensile strength tests were conducted in a universal testing machine (model DL 2000, EMIC, São José dos Pinhais, Brazil), in which two self-lock claws distant 15.0 mm between each other were placed with constant cross-head speed of 1.0 mm/s 1,5 (figure 5A). To calculate the tensile strength (in MPa), the maximum load tension (in N) was divided by the value of the area of the central portion of the sample (in mm2). The thickness of each sample used for the area calculation was the mean of the measuring at three points on the central section of the sample, performed with the aid of a digital caliper (model 799, Starret, Itu, Brazil) (figure 5B).
Statistical analysis
The data of the tensile strength test were submitted to one-way ANOVA and Tukey's test to compare the mean values. The level of significance was set at 5% (p < 0.05).
Results
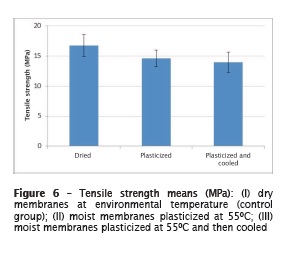
There were statistically significant differences among groups (p = 0.0022, figure 6). Group I (dry membranes) showed the highest tensile strength mean values (16.7 ± 1.9a MPa). There were no statistically significant differences between groups II (plasticized membranes: 14.6 ± 1.4b MPa) and III (plasticized and cooled membranes: 13.9 ± 1.7b MPa).
Discussion
The first use of polyglycolic acid (PGA) was in the construction of totally resorbable suture threads 9,12. Poly-lactic acid (PLA) is presented as distinct stereoisomers, dextro-gyrate (D) and levogyrous (L): L-PLA, D-PLA and DL-PLA 26. In this study, L-PLA was the polymer used to obtain PLGA because this is preferentially employed in materials requiring mechanical resistance and toughness 12.
PLGA copolymer membranes have been largely researched and studied because their degradation time can be controlled by the alteration of the concentrations of PLA and PGA copolymers and its molecular weight 19,22. Moreover, they are excellent mechanical barriers because they avoid the invasion of soft tissues and can be used as delivery system of drugs, skin replacements, vascular stents, and cell scaffolds 14. Also, they have the approval of Food and Drug Administration (FDA).
Sefat et al. 21 reported that dehydrated PLGA copolymer membranes present a hydrophobic feature, which makes difficult the cellular adhesion, therefore demanding a prior hydration before its use. The time recommended for this hydration is from 10 to 30 minutes in buffer phosphate-saline solution so that the process of cellular adhesion is more efficient 21.
However, according to the manufacturer, these membranes can be employed: (1) moist; (2) plasticized in heated solution; (3) cooled after plasticization. If the case does not require the molding of the membrane to the surgical site, the manufacturer advises only to place it over the receptor site for tissue regeneration and perform suture. If the case exhibits a surgical site of irregular morphology, it is advised to plasticize the membrane in solution heated at 55ºC, that is make it malleable, and adapt it over the site. Alternatively, the membrane can be cooled with saline solution after plasticization to memorize the desirable position.
The results of this present study showed that there were no stat ist ical ly signi f icant differences among tensile strength means after the plasticization regardless whether they had been cooled. Notwithstanding, the best results were achieved with the dried membrane. However, this is not advisable because it jeopardizes cellular aggregation.
Thus, the literature has reported the association of hydroxyapatite with PLGA copolymer to improve the mechanical properties of the membranes, achieving a force and hardness similar to that of the t issue surrounding the surgical site 1,21. Moreover, this association could neutralize the acids produced by the degradation of PLGA copolymer and promote better bone neoformation than that of pure polymers due to its cellular adhesion capacity 1,22,27,29.
With regard to cellular aggregation, most of the cells do not grow satisfactorily on the surface of PLGA membranes when compared with collagen membranes 4,11. Therefore, PLGA can be classified as a poor substrate for in vitro cellular growth 4,16. Another important factor to be reported is that the byproducts of PLGA copolymer, resulting from its degradation, are relatively strong acids (lactic acid and glycolic acid), which can accumulate on the surgical site and cause a late inflammatory response, thus negatively interfering in bone neoformation process 3,12,13,19,24.
Based on the aforementioned discussion, further studies are suggested aiming to analyze the degradation time and the residues coming from PLGA copolymer membrane after undergoing different humidity and temperature conditions similar to those of this study, since many studies have pointed out a late inflammatory response 3,12,13,19. Moreover, future studies are needed to verify the behavior of PLGA membranes as stem cell scaffolds by assessing the capacity of cellular adhesion to the substrate and cellular proliferation.
Conclusion
Based on the results obtained, it can be concluded that dried PLGA membranes show the greatest tensile strength compared with membranes only plasticized or cooled after plasticization.
Acknowledgment
The authors would like to thank Genius Biomateriais of Baumer S.A. for the PLGA copolymer membranes.
References
1. Asti A, Gastaldi G, Dorati R, Saindo E, Conti B, Visai L et al. Stem cells grown in osteogenic mediumon PLGA, PLGA/HA and t i tanium scaffolds for surgical applications. Bioinorg Chem Appl. 2010. [ Links ]
2. Bayat M, Momen-Heravi F, Marjani M, Motahhary P. A comparison of bone reconstruction following application of bone matrix gelatin and autogenous bone grafts to alveolar defects: an animal study. Journal of Cranio-Maxi l lo-Facial Surgery. 2010;38:288-92.
3. Bergsma EJ, Rozema FR, Bos RRM, Debruijn WC. Foreign body reaction to resorbable poly(Llactic) bone plates and screws used for the fixation of unstable zygomatic fractures. J Oral Maxillofac Surg. 1993;51:666-70.
4. Chen G, Liu D, Maruyama N, Ohgushi H, Tanaka J, Tateishi T. Cell adhesion of bone marrow cells, chondrocytes, ligament cells and synovial cells on a PLGA collagen hybrid mesh. Mater Sci Eng. 2004 Dec;24(6-8):867-73.
5. Chen G, Xia Y, Lu X, Zhou X, Zhang F, Gu N. Effects of surface functionalization of PLGA membranes for guided bone regeneration on proliferation and behavior of osteoblasts. J Biomed Mater Res. Part A. 2013;101A:4139-47.
6. Del Valle RA, Carvalho ML, Gonzales MR. Estudo do comportamento de enxerto ósseo com material doador obtido dos bancos dos tecidos músculo-esqueléticos. Revista de Odontologia da Universidade de São Paulo. 2006 May- Aug;18(2):189-94.
7. Fujihara K, Kotaki M, Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials. 2005;4139-47.
8. Griffin TJ, Cheung WS, Zavras AI, Damoulis PD. Postoperative complications following gingival augmentation procedures. J Periodontol. 2006;77:2070-9.
9. Hollander AP, Hatton PV. Methods in molecular biology: biopolymer methods in tissue engineering. Totowa: The Humana Press Inc; 2003. p. 1-10.
10. Kikuchi M, Koyama Y, Yamada T, Imamura Y, Okada T, Shirahma N et al. Development of guided bone regeneration membrane composed of b-tricalcium phosphate and poly(l-lactideco- glycolide-coe-caprolactone) composites. Biomaterials. 2004;5979-86.
11. Kim SY, Kanamori T, Noumi Y, Wang PC, Shinbo T. Preparation of porous poly(D,L-lactide) and poly(D,L-lactide-coglycolide) membranes by a phase inversion process and investigation of their morphological changes as cell culture scaffolds. J Appl Polym Sci. 2004;92:2082-92.
12. Kohn J, Abramson S, Langer R. Biomaterials science an introduction to materials in medicine. 2. ed. San Diego, CA: Elsevier; 2004. p. 115-20.
13. Martin C, Winet H, Bao JY. Acidity near eroding polylactidepolyglycolide in vitro and in vivo in rabbit tibial bone chambers. Biomaterials. 1996;17(24):2373-80.
14. Pan D, Liu LF, Wang BY. Factors affecting the guided tissue regeneration in periodontal tissue. Chin J Aesthet Med. 2005;14:509-14.
15. Pereira Neto ARL, Cruz ACC, Aragones A, Simões AMO, Souza JGO, Sella GC et al. Proliferation and viability of gingival human fibroblast cultured on membranes. In: IADR General Session, 2011, San Diego, California. Journal of Dental Research. 2011. v. 25.
16. Pezzin APT, Zavaglia CAC, Duek EAR. Estudo da degradação in vitro de blendas de poli(pdioxanona)/ poli(I-ácido láctico) (PPD/PLLA) preparadas por evaporação do solvente. Polímeros: Ciência e Tecnologia. 2002;12(4):285-94.
17. Pinto JGS, Ciprandi MTO, Aguiar RC, Lima PVP, Hernandez PAG, Silva Júnior AN. Enxerto autógeno x biomateriais no tratamento de fraturas e deformidades faciais – uma revisão de conceitos atuais. RFO. 2007 Sep-Dec;12(3):79-84.
18. Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. Journal of Prosthodontic Research. 2013;57:3-14.
19. Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioact i ve porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006 Jun;27(18):3413-31.
20. Seal BL, Otero TC, Panitch A. Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng: R: Rep. 2001;34:147-230.
21. Sefat F, Mckean R, Deshpande P, Ramachandran C, Hill CJ, Sangwan VS et al. Production, sterilization and storage of biodegradable electrospun PLGA membranes for delivery of limbal stem cells to the cornea. In: 3rd International Conference on Tissue Engineering; 2013; Leiria, Portugal. Elsevier; 2013. p. 101-16.
22. Song X, Ling F, Ma L, Yang C, Chen X. Electrospun hydroxyapatite grafted poly(L-lactide)/ poly(lactic-co-glycolic acid) nanofibers for guided bone regeneration membrane. Composites Science and Technology. 2013;79:8-14.
23. Stevens B, Yang Y, Mohandas A, Stucker B, Nguten KT. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res. Part B: Appl Biomater. 2008;85B:573-82.
24. Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004 Nov;25(26):5735-42.
25. Takechi M, Ohta K, Ninomiya Y, Tada M, Minami M, Takamoto M et al. 3-dimensional composite scaffolds consisting of apatite-PLGAatelocollagen for bone tissue engineering. Dent Mater J. 2012;31(3):465-71.
26. Tokiwa Y, Calabia BP, Ugwu CU, Aiba S. Biodegradability of plastics. Int J Mol Sci. 2009 Sep;10(9):3722-42.
27. Wu C, Zhang Y, Fan W, Ke X, Hu X, Zhou Y et al. CaSiO3 microstructure modulating the in vitro and in vivo bioactivity of poly(lactide-coglycolide) microspheres. J Biomed Mater Res A. 2011;98(1):122-31.
28. Zhang M. Biocompatible of materials. In: Shi D, Wang M, Zhang M, Clare A, Kasuga T, Liu Q, editors. Biomaterials and tissue engineering. Berlin/Heidelberg: Springer-Verlag; 2004.
29. Zhang P, Hong Z, Yu T, Chen X, Jing X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(Llactide). Biomaterials. 2009 Jan;30(1):58-70.
 Corresponding author:
Corresponding author:
Bárbara Pick Ornaghi
Rua Professor Pedro Viriato Parigot de Souza, n. 5.300 – Campo Comprido
CEP 81280-330 – Curitiba – PR – Brasil
E-mail: bpo@up.com.br
Received for publication: September 12, 2013
Accepted for publication: November 20, 2013













