Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.1 Joinville Jan./Mar. 2014
ORIGINAL RESEARCH ARTICLE
Analysis of salivary pH, flow rate, buffering capacity, concentrations of calcium, urea and total proteins in 2-8 years-old children with Down's syndrome
Gizele FrancoI; Rafaella SaabI; Luciani Variani PizzattoI; Maria Fernanda TorresII; Andréa Paula FregonezeIII; João Armando BrancherI
I Positivo University – Curitiba – PR – Brazil
II Federal University of Paraná – Curitiba – PR – Brazil
III Pontifical Catholic University – Curitiba – PR – Brazil
ABSTRACT
Introduction: Down syndrome (DS) is a genetic disorder caused by trisomy of chromosome 21. It is the most common chromosomal abnormality found in humans. Despite the motor difficulties and biofilm accumulation, individuals with DS have low caries prevalence. In this context it is assumed that saliva plays an important role in maintaining oral health. Objective: To evaluate the following salivary components: pH, buffering capacity and salivary flow volume in children with DS aging 2-8 years-old in the city of Curitiba (PR). Material and methods: Saliva samples were collected from 20 children with DS. The following parameters were evaluated: buffering capacity, flow rate, pH, and concentrations of calcium, urea and total proteins. Results: There was a normal distribution among the variables and the values observed were not statistically significant (p > 0.05). Conclusion: The results of this study revealed that there were no statistically significant differences in salivary flow, pH, buffering capacity, urea, calcium and total proteins in the subjects studied.
Keywords: Down syndrome; saliva; salivary flow.
Introduction
Down syndrome (DS) is a genetic disturb caused by the trisomy of chromosome 21 12. It is the most common chromosomal anomaly found in humans. In Brazil, at every 700 births, one child has born with DS, regardless of the race, gender or social class 6.
The term "syndrome" indicates the set of characteristics which identifies the carrier. In DS, the obvious signs are related to the motor and cognitive impairment with physical features characterized by rounded or flattened face, oblique eyes, strabismus, small nose with flattening of the tip, large tongue and short neck 16. Congenital heart diseases, recurrent respiratory infections, gastrointest inal disorders, neurological and endocrine alterations can also be seen 5.
Within oral cavity, narrow maxilla, high palate, delay and alteration in tooth eruption, and tooth agenesis are frequent. Clinically, it is evident the biofilm accumulation onto the teeth, a determinant factor for the beginning and progression of caries disease 24 and a high rate of periodontal disease, probably due to a low immunological response 20. Despite of the motor impairment and biofilm accumulation, DS individuals have low caries prevalence 7. In this context, it is believed that saliva plays and important role in maintaining oral health because of its organic and inorganic components influencing on the fragile balance of oral microbiota 13,25. Although the scientific literature has published many studies on saliva, this has received little attention by dentists. Therefore, the aim of this study was to verify the saliva of DS individuals from the city of Curitiba, Brazil.
Material and methods
This study was submitted and approved by the Ethical Committee in Research of Positivo University, under protocol number 480602/2012, according the Guideline number 196/96 of the Brazilian Health Council. Only the children whose parents or legal responsible person signed the free and clarified consent forms participated in the study.
Thus, 20 DS children, both genders, aged from 2 to 8 years-old, studying at the School of Special Education, Development and Stimulation (Cedae/Apae), in the city of Curitiba (PR, Brazil) participated in the study. Two examiners performed all clinical examinations, during the school period of the children, according to the international guidelines established by World Health Organization. All children exhibiting oral health problems were referred to the dental clinics of the Schools of Dentistry closer to the school.
The saliva samples of 20 children were col lected in a si lent env ironment, without interferences or external stimulations, with the aid of micropipette with disposable tips, without stimulus, for 10 minutes. Salivary flow was determined by dividing the volume collected by the time of aspiration. All saliva produced was stored into a sterile universal collector vial. The measurement of pH was performed with the aid of a portable pH meter (Digimed Analytical Instrumentation, DM 23). Salivary buffering capacity was determined with the aid of Caritest®–SL kit (Technew Comércio e Indústria Ltda.). For this purpose, 1 ml of the collected saliva was added to a f lask containing 3 ml of 0.005N HCl solution, and the reading of the samples strictly followed the manufacturer's instructions.
The salivary concentrat ions of calcium, urea and proteins were determined through colorimetric tests (Labtest Diagnóstica, Vista Alegre, MG, Brazil). The salivary biochemical tests were conducted always in triplicate. The obtained data were obtained by applying Kolmogorov- Smirnov test for normality, Levene test for homogeneity of variances and Student t test. The level of significance was set at 0.05.
Results
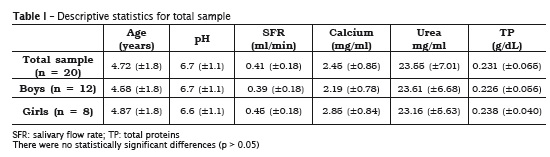
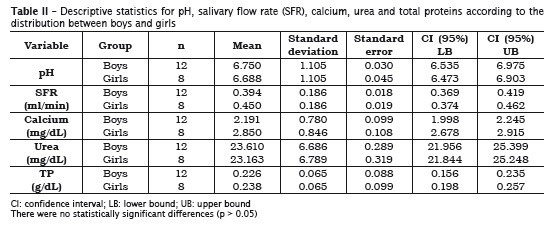
The analyzed variables showed normal dist r ibut ion because the va lues were not statistically significant (p > 0.05) (table I). Levene test exhibited that the variables were homogenous between genders without statistically significant differences (p > 0.05). The mean values of both genders were compared through Student t test for independent samples, without statistically significant differences (table II).
Discussion
The saliva plays important roles in the maintenance of the oral health because it can prevent bacterial invasion, growth and metabolism through different mechanisms 25. Also, it can modulate the bacterial adhesion to teeth and attenuate the deleterious effects of the production of metabolites by oral microbiota 23, due to its organic and inorganic components, contributing for oral health maintenance 11.
In this present study, 20 saliva samples of DS children aged from 2 to 8 years-old (12 boys and 8 girls) were verified. Salivary pH, flow, buffering capacity as well as calcium, urea and total proteins were assessed. There were no statistically significant differences for all variables between genders (p > 0.05).
Salivary flow is a very individualized measurement and varies according to circadian rhythm. It is known that the constant salivary flow can efficiently dilute and eliminate the products of the bacterial metabolism within oral cavity. Low salivary flow has been associated with high caries prevalence 28. The data found in this study confirmed the results from prior researches in which the mean salivary flow values were significantly smaller for DS individuals 21,26. In this context, special care should be given to DS individual, since low salivary flow, sugar consumption and the natural motor impairment can contribute for caries development.
pH did not show significant alteration (p > 0.05) and ranged from 6.7 (±1.1) for boys and 6.6 (±1.1) for girls. There is a consensus in the literature that oral pH varies from 6.8 to 7.2, in average, in the different world population, with little alterations, regardless of the age 11. When the analyzed sample is from nonstimulated saliva, pH tends to be low, about 5.6; however, pH increases when salivary flow is high 4. Prior studies have already demonstrated that salivary pH values from DS individuals were not statistically significant when compared with those without the syndrome 2,26.
Salivary buffering capacity is the ability of saliva in keeping stable oral pH, within the limits of normality, that is, it is the capacity of saliva in neutralizing the acids and/or bases within oral cavity, contributing to oral health 15. All studied individuals exhibited good buffering capacity.
Salivary proteins have many functions, among them, the bacterial aggregation 1,8; oxidation of hydrogen peroxide; antiviral, antimicrobial and antifungal activity. They also inhibit mineral precipitation and aids in remineralization 11. On the other hand, salivary collagenases can also account for the increase of periodontal disease in DS individuals 9. The measurement of proteins individually requires elaborated and laborious techniques and methodologies. Thus, in this present study, salivary total proteins were quantified. It was observed no statistically significant differences between boys and girls, with values found within the thresholds described in the literature for individuals without the syndrome.
Urea is an organic component and plays an important role in salivary biochemical because when metabolized by bacteria within the biofilm, there is the releasing of ammonia, which is capable of neutralizing the acids produced by the biofilm and assures certain immunity against caries 27. Previous studies have demonstrated the relationship between caries and salivary urea and found a smaller caries prevalence in individuals with chronic kidney disease than in normal people, probably because the higher concentration of salivary urea 18,19.
The urea concentration in total saliva of healthy individuals is of about 30 mg/dL 14. The urea elevation can indicate systemic alteration, mainly in the elderly 17 and nephropathic individuals 3. It is possible that the reduction in the salivary urea concentration is associated with morphofunctional changes in salivary glands. Therefore, further studies are necessary to prove scientifically the congenital anomalies involving these glands. The results obtained in this study on DS individuals revealed that the concentration of salivary urea in these children was 23.55 mg/dL (±7.01). Although there are not salivary urea values from DS children within this age range, the results were close to those of people without the syndrome. There were no statistically significant differences between boys and girls.
Although DS individuals have some motor impairment and consequently biofilm accumulation onto the teeth, caries prevalence is low. A possible rationale behind this is the high concentration of salivary calcium even with low salivary flow. High levels of salivary calcium determine a protector factor on the teeth 22. Calcium concentration is inf luenced according to the salivary f low value. According to Jenkins and Hargreaves 10, the higher the salivary flow, the higher the concentration of calcium ions within saliva. This present study revealed that despite of the reduced salivary flow, the levels of salivary calcium are high in DS individuals; however, this association was not statistically significant.
Conclusion
The salivary variables studied have been largely used to determine the risk of oral diseases, specially caries and periodontal disease. None of these variables showed statistically significant differences when compared with data of the literature from individuals without DS. The results revealed that there were no statistically significant differences in salivary flow, pH, buffering capacity, urea, calcium, and total proteins between boys and girls.
References
1. Antti SA, Tenovuo J. Association between mother-infant salivary contacts and caries resistance in children: a cohort study. Pediatric Dentistry. 1994;16(2):9-14. [ Links ]
2. Cogulu D, Sabah E, Kutukculer N, Ozkinay F. Evaluation of the relationship between caries indices and salivary secretory IgA, salivary pH, buffering capacity and flow rate in children with Down's syndrome. Arch Oral Biol. 2006;51(1):23-8.
3. Courts FJ, Tapley PM. Relationship of salivary urea to caries incidence in CRF patients. J Dent Res. 1984;63(2):184-98.
4. Dawes C, Jenkins GN. The effects of different stimuli on the composition of saliva in man. J Physiol. 1964;170:86-100.
5. Desai SS. Down syndrome: a review of the literature. Oral Surgery Oral Medicine Oral Pathology Oral Radiology. 1997;84:279-85.
6. Ministério da Saúde. Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Ações Programáticas Estratégicas. Diretrizes de atenção à pessoa com síndrome de Down. Brasília; 2012.
7. Fiorati SM, Spósito RA, Borsatto MC. Prevalência de cárie dentária e doença periodontal em pacientes com síndrome de Down. Odontol 2000. 1999;3(2):58-62.
8. Gahnberg L, Krasse B. Salivary immunoglobulin A antibodies and recovery from challenge of Streptococcus mutans after oral administration of Streptococcus mutans vaccine in humans. Infect Immun. 1983(39):514-9.
9. Halinen S, Sorsa T, Ding Y, Ingman T, Salo T, Konttinen YT et al. Characterization of matrix metalloproteinase (MMP-8 and -9) activities in the saliva and in gingival crevicular fluid of children with Down's syndrome. Journal of Periodontology. 1986(67):748-54.
10. Jenkins GN, Hargreaves JA. Effect of eating cheese on Ca and P concentrations of whole mouth saliva and plaque. Caries Res. 1989;23(3):159-64.
11. Kidd E, Fejerskov O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. Journal of Dental Research. 2004(83):35-8.
12. Lejeune J, Turpin R, Gautier M. Mongolism: a chromosomal disease (trisomy). Bulletin d e l 'Ac a d èmi e Na t i o n a l e d e Mè d e c i n e . 1959;143:256-65.
13. Lenander-Lumikari M, Loimaranta V. Saliva and dental caries. Adv Dent Res. 2000;14:40-7.
14. Macpherson LM, Dawes C. Urea concentration in minor mucous gland secretions and the effect of salivary film velocity on urea metabolism by Streptococcus vestibularis in an artificial plaque. J Periodontal Res. 1991;26(5):395-401.
15. Mandel ID. Impact of saliva on dental caries. Compendium of Continuing Education in Dentistry. 1989(1):476-81.
16. Moraes ME, Moraes LC, Dotto GN, Dotto PP, Santos LR. Dental anomalies in patients with Down syndrome. Braz Dent J. 2007;18(4):346-50.
17. Pajukoski H, Meurman JH, Snellman-Gröhn S, Keinänen S, Sulkava R. Salivary flow and composition in elderly patients referred to an acute care geriatric ward. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(3):265-71.
18. Peterson S, Woodhead J, Crall J. Caries resistance in children with chronic renal failure: plaque pH, salivary pH, and salivary composition. Pediatr Res. 1985;19(8):796-9.
19. Ruchi A, Bhumi S. Estimation of salivary urea levels and its relation with dental caries in children with chronic renal failure. Journal of Oral Health Research. 2010;1(2):70-5.
20. Santos P. Pacientes com síndrome de Down apresentam doença periodontal precoce. Rev Sobrape. 2003;81:53-7.
21. Siqueira WL, Bermejo PR, Mustacchi Z, Nicolau J. Buffer capacity, pH, and flow rate in saliva of children aged 2-60 months with Down syndrome. Clin Oral Investig. 2004;9(1):26-9.
22. Sewon L, Makela M. A study of the possible correlation of high salivary calcium levels with periodontal and dental conditions in young adults. Arch Oral Biol. 1990;35:211-2.
23. Tenovuo J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactotransferrin in xerostomia: efficacy and safety. Oral Diseases. 2002;8:23-9.
24. Van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73(3):672-81.
25. Van Nieuw Amerongen A, Bolscher J, Veerman EC. Salivary proteins: protective and diagnostic value in cariology? Caries Research. 2004;38:247-53.
26. Yarat A, Akyüz S, Koç L, Erdem H, Emekli N. Salivary sialic acid, protein, salivary flow rate, pH, buffering capacity and caries indices in subjects with Down's syndrome. J Dent. 1999;27(2):115-8.
27. Zabokova E, Sotirovska IA, Ambarkova V. Correlation between salivary urea level and dental caries. Prilozi. 2012;33(1):289-302.
28. Zijinge V, Van Leewen B, Degener J, Abbas F, Thurnheer T. Oral biofilm architecture on natural teeth. 2010;5:1-9.
 Corresponding author:
Corresponding author:
João Armando Brancher
Rua Professor Pedro Viriato Parigot de Souza, n. 5.300 – Campo Comprido
CEP 81280-330 – Curitiba – PR – Brasil
E-mail: brancher@up.com.br
Received for publication: October 14, 2013
Accepted for publication: November 18, 2013













