Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.1 Joinville Jan./Mar. 2014
LITERATURE REVIEW ARTICLE
Cell adhesion in bone grafts associated to nanotechnology: a systematic review
Haroldo Gurgel Mota-FilhoI; Amanda Alencar CabralII; Diego Moura SoaresIII; Fernanda GinaniIV; Carlos Augusto Galvão BarbozaIV
I Graduation Course in Dentistry, Federal University of Rio Grande do Norte – Natal – RN – Brazil
II Graduation Course in Biological Sciences, Federal University of Rio Grande do Norte – Natal – RN – Brazil
III Program of Post-Graduation in Dentistry, Federal University of Pernambuco – Natal – RN – Brazil
IV Program of Post-Graduation in Oral Pathology, Federal University of Rio Grande do Norte – Natal – RN – Brazil
ABSTRACT
Introduction: Tissue engineering aims at the development of biological substitutes that can restore, maintain, or improve the functionality of damaged tissue or organs. To this end, molecular and cellular interactions may influence the tissue reactions to biomaterials. In order to be effective and integrated to the receiving area, the bone graft is required to allow a strong cell adhesion, interacting with several molecules to induce migration, differentiation, and thus the mineralization of the new bone on the graft. These cell adhesion molecules (CAM) will mediate the contact between two cells or between cells and the extracellular matrix, an essential process to the success of the implant. Objective: This paper is a systematic review of the literature on the mechanisms of cell adhesion to bone grafts associated to nanotechnology, describing the importance and the role of those molecules in the adhesion and thus in tissue regeneration. Literature review: After the use of search strategies, 18 articles that describe processes of cell adhesion to bone grafts were selected. Results: The main reported mechanisms involve cell adhesion molecules (CAMs) and extracellular matrix components. Conclusion: Several molecules are involved in the process of cell adhesion to bone grafts, highlighting the role of integrins, the focal adhesion mechanism, the influence of the collagen matrix, and the activity of alkaline phosphatase in bone matrix formation. Accurate identification of these mechanisms of cell adhesion is essential for further advancement in tissue engineering, such as the production of biological bone substitutes that achieve a better clinical outcome.
Keywords: cell adhesion; bone and bones; nanotechnology.
Introduction
Bone regeneration is a complex and continuous process aiming at the anatomic and functional restoration and it can demand the use of biomaterials to promote a fast bone formation. Likely other tissues, many events occur when a given biomaterial is in contact with the biological bone environment, with molecular and cellular interactions influencing on the tissue features surrounding the biomaterial. In its presence, growth factors either adsorb or moisten the surface of the bone substitutes, promoting an adequate integration with the host bone 13.
Tissue engineering is a strategy very used to obtain functional repairing through the development of biological substitutes that can restore, keep or substitute damaged tissues or organs 31, through the combination of scaffolds biocompatible with live cells and/or bioactive molecules 19. In this procedure it is possible to use stem cells obtained from different sources.
The use of nanotechnology plays an important role in tissue engineering because the properties that this technology adds to the material, such as the greater surface area and greater roughness, improve the physical-chemical properties which mimic those of the natural tissues and organs 31. These properties will act mediating the action of cell adhesion proteins, regulating cell behavior and causing tissue regeneration. Within bone tissue, the use of nano-modified scaffolds is a promising alternative aiming to accelerate the repair process and to reestablish the height and volume of the bone lost. Notwithstanding, to achieve the effectiveness and integration of the grafted tissue to the receptor site, it is necessary a strong cell adhesion, so that it demands many molecules interaction to induce cell differentiation and the bone matrix mineralization formed onto the graft. Some of these molecules are glycoproteins expressed on the cell surface, socalled cell adhesion molecules (CAM), which mediate either the contact between two cells or the contact among cells and the extracellular matrix, being therefore of fundamental importance for adhesion 3. CAMs have been classified into: cadherins, the immunoglobulin superfamily, integrins and selectins. To occur the adhesion of CAMs to the biomaterial, it is necessary their interaction with some components of extracellular matrix, such as proteoglycans, collagen and proteins 23.
Many of the properties giving biocompatibility to materials are closer related to the reaction of cells during contact, mainly in adhesion to their surface. The first interaction phase between the cell and biomaterial is characterized by events, such as approximation, adhesion and "spreading". The quality of this first phase will influence on the cell capacity to proliferate and differentiate in contact with the grafted material. This is essential for graft effectiveness, in order to establish a mechanically solid surface, by the complete fusion between the material and bone tissue and without the presence of a fibrous interface 3.
The aim of this study was to analyze the most updated studies on the mechanisms of cell adhesion to bone grafts using nanotechnology by modifying the surface that potentiates these processes. Also, it is described the importance and role of CAMs in cell adhesion, and consequently in tissue regeneration.
Literature review
The electronic search of the studies was executed until the ending of March of 2012, within journals available on PubMed/Medline database and published from 2007 to 2011. To perform the search, the search tool «advanced», available at NCBI site, was used by combining the descriptors All fields «nanotechnology and tissue engineering» and Date publication «2007/01/01 to 2011/12/31».
Inclusion criteria comprised the use of studies which the complete text could be accessed, in English language, classified as experimental studies with main objective of the use of nanotechnology applied to biomaterials for bone grafts, which described the process of cell adhesion.
The selection of the studies began by the evaluation of their titles identified by the application of the search strategy. The studies whose titles did not meet the aim of this present systematic review were excluded. All other studies were pre-selected and had their abstracts analyzed. Both the studies whose abstracts met the inclusion criteria and those which the abstracts did not provide enough data had their complete text analyzed. After the reading of the complete text, the studies describing experiments on other tissues or those which adhesion mechanism were not clear were excluded. After the application of the search strategy, 781 studies displaying the used terms were found. By applying a careful analysis and exclusion, 18 studies were selected, according to figure 1.
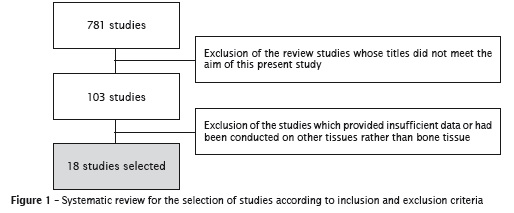
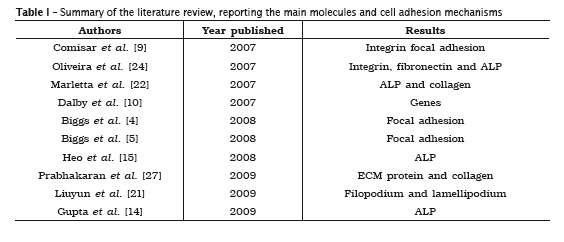
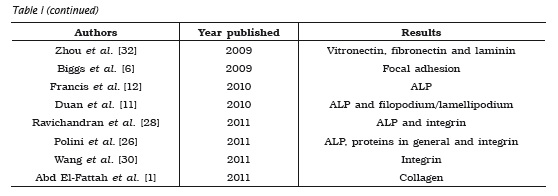
Many molecules and mechanisms related to the processes of cell adhesion in bone grafts were reported by the articles selected. Among the processes described, it could be observed an emphasis on the role of integrins9,24,26,27,30, alkaline phosphatase (ALP) 11,12,15,22,24,26,28 and on the mechanism of focal adhesion 4-6,9. These three mechanisms were the most cited because they have fundamental roles in the success and evaluation of adhesion, since integrins have significant influence on osteoblastic cell adhesion, ALP provides the differentiation degree of these cells, and focal adhesion acts as anchoring structure. The other molecules and mechanisms described are summarized in table I.
Generally, the processes of adhesion described by the authors involved some cell-to-cell and cell-tomatrix adhesion molecules (table II). Among these processes, filopodium is emphasized because it is vital for the establishment of cell-to-cell contact in epithelial cells and it is also involved in the metastasis of neoplastic cells 16, and lamellipodium, which has an important function in cell migration, including embryonic development, inflammation and metastasis of malign cells 25.

Discussion
One of the characterist ics of adhesion molecules is their capacity of interacting with specific ligands, which can be present in the cell membrane or extracellular matrix (ECM) 3. The main molecule of cell adhesion described by many authors selected in this study is from integrin family 9,24,26,28,30. These molecules account for either cell-to-cell or cell-to-matrix adhesion. Integrins are located in the interface between intra-and extracellular medium and consequently can translate the sings from external to internal medium, promoting the adhesion, spreading or migration of cells, therefore regulating the cell growth and differentiation 3,18.
According to the data searched in the literature, it is inferred that integrins have a significant inf luence on the adhesion of osteoblastic cells 3,9, because its amount can affect the degree of maturity of focal adhesion, the recruitment or proteins from the cytoskeleton and still influence the signaling of the molecules 9.
It must have a primary cell-to-matrix interaction to occur cell differentiation. This process is known as focal adhesion, characterized by a strong contact through integrin receptors, between the cell and the material to which the adhesion will take place 23. Thus, the site of focal adhesion acts as an anchoring structure 23 which will influence on the formation of a fibronectin matrix 17. Concerning to ECM, the collagen is one of the main components cited by the authors 1,22,27, since it is an essential candidate to compose ECM due to its properties of providing mechanical resistance to the tissue and inf luence on cell adhesion and differentiation 23.
The studies selected also cited other essential components of ECM which also acts in cell adhesion, such as laminin and vitronectin 22 and fibronectin 24,32.
According to Iilic et al. 17, fibronectin/ vitronectin are fundamental for focal adhesion because they have the capacity of communicating with integrins through a sequence of amino acids known as RGD (arginine, glycine and asparagine), allowing the cell "anchoring". Cowles et al. 8 also emphasized such importance and related it with the initial stages of osteoblast differentiation. According to Carvalho et al. 7, cell adhesion capacity is related with the presence of collagen and fibronectin/vitronectin within ECM, while laminin acts as a limiting factor of cell adhesion within ECM. Therefore, it can be affirmed that these proteins are essential for culture cell adhesion and consequently for tissue development. Additionally, other proteins were reported in the studies 26,27, but they were not specified by the authors.
Some authors observed the lamellipodium and filopodium formation as fundamental mechanisms for cell anchoring and proliferation, because they have been used to aid in cell adhesion and elongation 11,21.
Other important mechanism of cell adhesion is ALP 11,12,15,22,24,26,28, which participating in the cleavage of organic phosphate esters and it is a fundamental component of bone matrix because is important during the formation of hydroxyapatite and calcium crystals 2. The expression of ALP activity in the cells shows the bone capacity of forming osteoblast regardless of scaffolds 12 and providing the degree of osteoblastic differentiation 15.
Concerning to gene expression described in the study of Dalby et al. 10, this has been a fundamental factor to occur all adhesion, growth, and differentiation of the cells. A better understanding of these mechanisms of guidance and cell-to-cell or cell-to-matrix interaction is essential for the construction of biomaterials with nano-specific properties to provoke a better cell response and consequently a greater applicability in tissue engineering 29.
To compare the results better, the standardization of the studies is necessary so that the same adhesion analyses are employed and the results are observed in the same design (in vitro / in vivo). The limitation in this type of study is the comparison of the quantitative and qualitative data due to the variety of protocols which makes difficult the correlation among the studies.
Conclusion
The process of cell adhesion in bone grafts occur through many molecules and cell mechanisms (integrins, focal adhesion, collagen matrix and ALP). To know these cell mechanisms is fundamental to identify and quantify a good cell adhesion, consequently leading to great advancements in tissue engineering.
References
1. Abd El-Fattah HMDDS, Helmy YBDS, El-Kholy, Marie M. In vivo animal histomorphometric study for evaluating biocompatibility and osteointegration of nano-hydroxyapatite as biomaterials in tissue engineering. Journal of the Egyptian Nat Cancer Inst. 2011;22(4):241-50.
2. Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232-5.
3. Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667-81.
4. Biggs MJP, Richards RG, Gadegaard N, Mcmurray RJ, Affrissman S, Wilkinson CDW et al. Interactions with nanoscale topography: adhesion quantification and signal transduction in cells ofosteogenic and multipotent lineage. J Biomed Mater Res A. 2008;91(1):195-208.
5. Biggs MJP, Richards RG, McFarlane CDW, Wilkinson CDW, Oreffo ROC, Dalby MJ. Adhesion formation of primary human osteoblasts and the functional response of mesenchymal stem cells to 330 nm deep microgrooves. J R Soc Interface. 2008;5:1231-42.
6. Biggs MJP, Richards RG, Gadegaard N, Wilkinson CDW, Oreffo ROC, Dalby MJ. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signaling in STRO-1þ enriched skeletal stem cells. Biomaterials. 2009;30:5094-103.
7. Carvalho MV, Alves PM, Oliveira RS, Ramalho LS, Queiroz LMG. Estudo imuno-histoquímico da tenascina-C e fibronectina em lesões proliferativas não-neo-plásicas de mucosa oral. Odontol Clín- Científ. 2009;8(4):353-7.
8. Cowles EA, Derome ME, Pastizzo G, Brailey LL, Gronowicz GA. Mineralization and the expression of matrix proteins during in vivo bone development. Calcif Tissue Int. 1998;62(1):74-82.
9. Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials. 2007;28:4409-17.
10. Dalby MJ, Gadegaard NJ, Tare R, Andar A, Riehle MO, Herzyk P et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Materials. 2007;6:997-1003.
11. Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomaterialia. 2010;6(12):4495-505.
12. Francis L, Venugopal J, Prabhakaran MP, Thavasi V, Marsano E, Ramakrishna S. Simultaneous e l e c t rospin– e l e c t rospray ed biocomposi t e nanofibrous scaffolds for bone tissue regeneration. Acta Biomaterialia. 2010;6(10):4100-9.
13. Garofalo GS. Autogenous, allogenetic and xenogenetic grafts for maxillary sinus elevation: literature review, current status and prospects. Minerva Stomatol. 2007;56(7-8):373-92.
14. Gupta D, Venugopal J, Mitra S, Dev VRG, Ramakrishna S. Nanostructured biocomposite substrates by electrospinning and electrospraying for the mineralization of osteoblasts. Biomaterials. 2009;30(11):2085-94.
15. Heo SJ, Kim SE, Wei J, Kim DH, Hyun YT, Yun HS et al. In vitro and animal study of novel nanohydroxyapatite= poly(e-Caprolactone) composite scaffolds fabricated by layer manufacturing process. Tissue Eng Part A. 2008;15(5):977-89.
16. Hoffmann B, Schafer C. Filopodial focal complexes direct adhesion and force generation towards filopodia outgrowth. Cell Adhesion & Migration. 2010;4(2):190-3.
17. Iilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomase-Vic N, Han Q et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117(Pt 2):177-87.
18. Krauser A, Cowles EA, Gronowicz G. Integrinmediated signaling in osteoblasts on titanium implant materials. J Biomed Mater. Res. 2000;52 (4):738-47.
19. Kubinová S, Syková E. Nanotechnologies in regenerative medicine. Minimally Invasive Therapy. 2010;19:144-56.
20. Lian JB, Stein GS. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Lowa Orthop J. 1995;15:118-40.
21. Liuyun J, Yubao L, Chengdong X. Preparation and biological properties of a novel composite scaf fold of nano-hydroxyapat i te/chi tosan/ carboxymethyl cellulose for bone tissue engineering. Journal of Biomedical Science. 2009;16(6):1-10.
22. Marletta G, Ciapetti G, Satriano C, Perut F, Salerno M, Baldini M. Improved osteogenic differentiation of human marrow stromal cells cultured on ion-induced chemically structured polye- caprolactone. Biomaterials. 2007;28(6):1132-40.
23. Meyer U, Buchter A, Wiesmann HP, Joos U, Jones DB. Basic reactions of osteoblasts on structured material surfaces. Eur Cell Mater. 2005;9:39-49.
24. Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. Journal of Biomedical Materials Research Part. 2007;80(3):554-64.
25. Pinco KA, He W, Yang JT. α4β1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Molecular Biology of the Cell. 2002;13:3203-17.
26. Polini A, Pisignano D, Parodi M, Quarto R, Scaglione S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE. 2011;6(10):e26211.
27. Prabhakaran MP, Venugopal J, Ramakrishna S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomaterialia. 2009;5(8):2884-93.
28. Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nano fibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials. 2011;33(3):846-55.
29. Toh YC, Ng S, Khong YM, Zhang X, Zhum Y, Lin PC et al. Cellular responses to a nanofibrous environment. Nanotoday. 2006;1(3):34-43.
30. Wang G, Zheng L, Zhao H, Miao J, Sun C, Liu H et al. Construction of a fluorescent nanostructured chitosan-hydroxyapat ite scaffold by nanocryst allon induced biomimetic mineralization and its cell biocompatibility. ACS Appl Mater Interfaces. 2011;3:1692-701.
31. Zhang L, Wbster T. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4(1):66-80.
32. Zhou WY, Guo B, Liu M, Liao R, Rabie ABM, Jia D. Poly(vinyl alcohol)/halloysite nanotubes bionanocomposite films: properties and in vitro osteoblasts and fibroblasts response. J Biomed Mater Res A. 2009;93(4):1574-8.
 Corresponding author:
Corresponding author:
Carlos Augusto Galvão Barboza
Universidade Federal do Rio Grande do Norte
Centro de Biociências – Departamento de Morfologia
Av. Salgado Filho, n. 3.000 – Campus Universitário
CEP 59072-970 – Natal – RN – Brasil
E-mail: cbarboza@cb.ufrn.br
Received for publication: July 18, 2013
Accepted for publication: November 11, 2013













